Nickel-Copper Oxide Catalysts Deposited on Stainless Steel Meshes by Plasma Jet Sputtering: Comparison with Granular Analogues and Synergistic Effect in VOC Oxidation
Abstract
:1. Introduction
2. Results
2.1. Composition of the Catalysts
2.2. Powder X-ray Diffraction
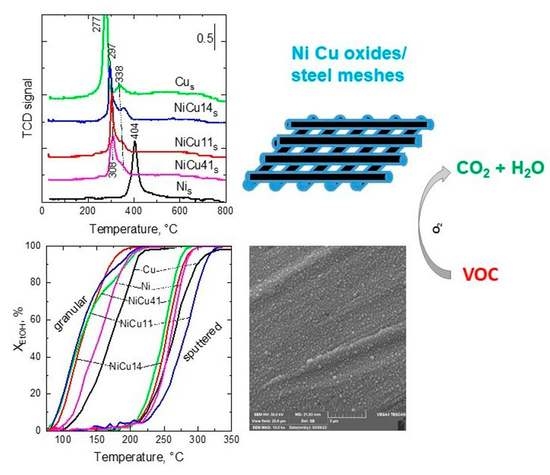
2.3. Temperature-Programmed Reduction
2.4. Nitrogen Adsorption Measurements
2.5. Raman Spectroscopy
2.6. X-ray Photoelectron Spectroscopy
2.7. Catalytic Performance
3. Discussion
4. Experimental
4.1. Preparation of Catalysts
4.1.1. Granular Ni-Cu Oxide Catalysts
4.1.2. Sputtered Ni-Cu Oxide Catalysts
4.2. Catalyst Characterization
4.3. Catalytic Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Ding, H.; Pan, W.; Mu, X.; Qiu, K.; Ma, J.; Zhao, Y.; Song, J.; Zhang, Z. Research Progress of a Composite Metal Oxide Catalyst for VOC Degradation. Environ. Sci. Technol. 2022, 56, 9220–9236. [Google Scholar] [CrossRef]
- Topka, P.; Jirátová, K.; Dvořáková, M.; Balabánová, J.; Koštejn, M.; Kovanda, F. Hydrothermal deposition as a novel method for the preparation of Co–Mn mixed oxide catalysts supported on stainless steel meshes: Application to VOC oxidation. Environ. Sci. Pollut. Res. 2022, 29, 5172–5183. [Google Scholar] [CrossRef]
- Chen, L.C.; Lin, S.D. The ethanol steam reforming over Cu-Ni/SiO2 catalysts: Effect of Cu/Ni ratio. Appl. Catal. B Environ. 2011, 106, 639–649. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. Synergism studies on alumina-supported copper-nickel catalysts towards furfural and 5-hydroxymethylfurfural hydrogenation. J. Mol. Catal. A Chem. 2017, 426, 244–256. [Google Scholar] [CrossRef]
- Yen, H.; Kleitz, F. High-performance solid catalysts for H2 generation from ammonia borane: Progress through synergetic Cu–Ni interactions. J. Mater. Chem. A 2013, 1, 14790–14796. [Google Scholar] [CrossRef]
- Lv, H.; Sun, H. Foamlike NiO/CuO Nanocomposites with Superior Catalytic Activity toward the Reduction of 4-Nitrophenol. ACS Omega 2020, 5, 11324–11332. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, G.; Yao, X.; Liu, B. A comparison of NiO–CuO–CeO2 composite catalysts prepared via different methods for CO oxidation. J. Solid State Chem. 2020, 292, 121697. [Google Scholar] [CrossRef]
- Olejníček, J.; Šmíd, J.; Perekrestov, R.; Kšírová, P.; Rathouský, J.; Kohout, M.; Dvořáková, M.; Kment, Š.; Jurek, K.; Čada, M.; et al. Co3O4 thin films prepared by hollow cathode discharge. Surf. Coat. Technol. 2019, 366, 303–310. [Google Scholar] [CrossRef]
- Jirátová, K.; Perekrestov, R.; Dvořáková, M.; Balabánová, J.; Koštejn, M.; Veselý, M.; Čada, M.; Topka, P.; Pokorná, D.; Hubička, Z.; et al. Modification of Cobalt Oxide Electrochemically Deposited on Stainless Steel Meshes with Co-Mn Thin Films Prepared by Magnetron Sputtering: Effect of Preparation Method and Application to Ethanol Oxidation. Catalysts 2021, 11, 1453. [Google Scholar] [CrossRef]
- Hubička, Z.; Kment, Š.; Olejníček, J.; Čada, M.; Kubart, T.; Brunclíková, M.; Kšírová, P.; Adámek, P.; Remeš, Z. Deposition of hematite Fe2O3 thin film by DC pulsed magnetron and DC pulsed hollow cathode sputtering system. Thin Solid Films 2013, 549, 184–191. [Google Scholar] [CrossRef]
- Stoyanova, D.; Michailova, I.; Radev, D.; Ivanov, G.; Mehandjiev, D. Catalytic oxidation of CO by copper-nickel oxides supported on γ-alumina. C. R. L’académie Bulg. Sci. Sci. Mathématiques Nat. 2017, 70, 1095–1102. [Google Scholar]
- Fedorov, A.V.; Kukushkin, R.G.; Yeletsky, P.M.; Bulavchenko, O.A.; Chesalov, Y.A.; Yakovlev, V.A. Temperature-programmed reduction of model CuO, NiO and mixed CuO–NiO catalysts with hydrogen. J. Alloys Compd. 2020, 844, 156135. [Google Scholar] [CrossRef]
- Abo Zeid, E.F.; Nassar, A.M.; Hussein, M.A.; Alam, M.M.; Asiri, A.M.; Hegazy, H.H.; Rahman, M.M. Mixed oxides CuO-NiO fabricated for selective detection of 2-Aminophenol by electrochemical approach. J. Mater. Res. Technol. 2019, 9, 1457–1467. [Google Scholar] [CrossRef]
- Oluku, I.; Ibrahim, H.; Idem, R. Ternary oxide-supported bimetallic nickel-copper catalysts for a single step high temperature water gas shift of biogas reformate. Fuel 2018, 234, 1238–1258. [Google Scholar] [CrossRef]
- Radev, D.; Michailova, I.; Mechandjiev, D. Mechanically assisted synthesis of mixed nickel-copper oxides. C. R. L’académie Bulg. Sci. Sci. Mathématiques Nat. 2019, 72, 604. [Google Scholar] [CrossRef]
- Paryjczak, T.; Rynkowski, J.; Krzyzanowski, K. Temperature-programmed reduction of CuO−NiO/Al2O3 catalysts. React Kinet. Catal. Lett. 1982, 21, 295–298. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Patcas, F.; Hahn, T. A spillover approach to oxidation catalysis over copper and manganese mixed oxides. Chem. Eng. Process. 1999, 38, 563–569. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hu, X.; Wu, R.; Zhao, Y. Preparation and characterization of Cu–Mn composite oxides in N2O decomposition. Reac. Kinet. Mech. Cat. 2020, 129, 165–179. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Li, W.; Zhou, L.; Lan, Y.; Li, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Suib, S.L. New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bose, P.; Ghosh, S.; Basak, S.; Naskar, M.K. A facile synthesis of mesoporous NiO nanosheets and their application in CO oxidation. J. Asian Ceram. Soc. 2016, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, Y.; Xu, C.; Zheng, C.; Wang, G. Synthesis of NiO nanorods by a novel simple precursor thermal decomposition approach. Chem. Phys. Lett. 2002, 362, 119–122. [Google Scholar] [CrossRef]
- Song, L.X.; Yang, Z.K.; Teng, Y.; Xia, J.; Du, P. Nickel oxide nanoflowers: Formation, structure, magnetic property and adsorptive performance towards organic dyes and heavy metal ions. J. Mater. Chem. A 2013, 1, 8731–8736. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pars, M. Raman scattering in nanosized nickel oxide. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.K.; Verma, S.; Rath, S. Magnetic-order induced effects in nanocrystalline NiO probed by Raman spectroscopy. Phys. Rev. B 2020, 102, 024423. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Puust, L.; Grabis, J. Magnon and phonon excitations in nanosized NiO. Latv. J. Phys. Tech. Sci. 2019, 56, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.S.; Venugopalan, V.P.; Arunya, D.D.; Dhara, S.; Pandiyan, R.; Tyagi, A.K. Antibiofilm Activity of Nano sized CuO. In Proceedings of the International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), Chennai, India, 28–30 November 2011; pp. 580–583. [Google Scholar] [CrossRef]
- Ciobotea-Barbu, O.C.; Ciobotaru, I.A.; Vaireanu, D.I.; Dumitras, D.G.; Nicolae, C. XRD, Raman and SEM surface analysis on Ni-Cu electrodeposited layers. J. Optoelectron. Adv. Mater. 2019, 21, 536–540. Available online: https://joam.inoe.ro/articles/xrd-raman-and-sem-surface-analysis-on-ni-cu-electrodeposited-layers/fulltext (accessed on 23 February 2023).
- Niaura, G. Surface-enhanced Raman spectroscopic observation of two kinds of adsorbed OH− ions at copper electrode. Electrochim. Acta 2000, 45, 3507–3519. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321. [Google Scholar] [CrossRef] [Green Version]
- Chou, M.H.; Liu, S.B.; Huang, C.Y.; Wu, S.Y.; Cheng, C.L. Confocal Raman spectroscopic mapping studies on a single CuO nanowire. Appl. Surf. Sci. 2008, 254, 7539–7543. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart RStC. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Kaštánek, F.; Topka, P.; Soukup, K.; Maléterová, Y.; Demnerová, K.; Kaštánek, P.; Šolcová, O. Remediation of Contaminated Soils by Thermal Desorption; Effect of Benzoyl Peroxide Addition. J. Clean Prod. 2016, 125, 309–313. [Google Scholar] [CrossRef]
- Gaálová, J.; Topka, P.; Kaluža, L.; Soukup, K.; Barbier, J., Jr. Effect of gold loading on ceria-zirconia support in total oxidation of VOCs. Catal. Today 2019, 333, 190–195. [Google Scholar] [CrossRef]
- De Waele, J.; Galvita, V.V.; Poelman, H.; Gabrovska, M.; Nikolova, D.; Damyanova, S.; Thybaut, J.W. Ethanol dehydrogenation over Cu catalysts promoted with Ni: Stability control. Appl. Catal. A 2020, 591, 117401. [Google Scholar] [CrossRef]
- Sun, L.; Deng, Y.; Yang, Y.; Xu, Z.; Xie, K.; Liao, L. Preparation and catalytic activity of magnetic bimetallic nickel/copper nanowires. RSC Adv. 2017, 7, 17781–17787. [Google Scholar] [CrossRef] [Green Version]
- Cross, A.; Miller, J.T.; Danghyan, V.; Mukasyan, A.S.; Wolf, E.E. Highly active and stable Ni-Cu supported catalysts prepared by combustion synthesis for hydrogen production from ethanol. Appl. Catal. A 2019, 572, 124–133. [Google Scholar] [CrossRef]
- Kumar, A.; Cross, A.; Manukyan, K.; Bhosale, R.R.; van den Broeke, L.J.P.; Miller, J.T.; Mukasyan, A.S.; Wolf, E.E. Combustion synthesis of copper–nickel catalysts for hydrogen production from ethanol. Chem. Eng. J. 2015, 278, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Topka, P.; Dvořáková, M.; Kšírová, P.; Perekrestov, R.; Čada, M.; Balabánová, J.; Koštejn, M.; Jirátová, K.; Kovanda, F. Structured cobalt oxide catalysts for VOC abatement: The effect of preparation method. Environ. Sci. Pollut. Res. 2020, 27, 7608–7617. [Google Scholar] [CrossRef]
- Matějová, L.; Topka, P.; Jirátová, K.; Šolcová, O. Total oxidation of model volatile organic compounds over some commercial catalysts. Appl. Catal. A 2012, 443, 40–49. [Google Scholar] [CrossRef]
- Dvořáková, M.; Perekrestov, R.; Kšírová, P.; Balabánová, J.; Jirátová, K.; Maixner, J.; Topka, P.; Rathouský, J.; Koštejn, M.; Čada, M.; et al. Preparation of cobalt oxide catalysts on stainless steel wire mesh by combination of magnetron sputtering and electrochemical deposition. Catal. Today 2019, 334, 13–23. [Google Scholar] [CrossRef]
- Topka, P.; Jirátová, K.; Soukup, K.; Goliáš, J. Device for Measuring Specific Surface of Large Samples, Method of Measurement and Its Use. Czech Patent CZ308606, 30 December 2020. [Google Scholar]











| Sample | Ni | Cu | Na | Ni:CuAAS |
|---|---|---|---|---|
| Cug | - | 77.2 | 0.2 | 0:1 |
| NiCu14g | 14.4 | 55.6 | 0.4 | 1:3.7 |
| NiCu11g | 36.6 | 36.6 | 0.2 | 1:0.9 |
| NiCu41g | 58.0 | 16.2 | 0.1 | 4:1.0 |
| Nig | 77.1 | - | 0.4 | 1:0 |
| Sample | Ni a | Fe a | Ni Correction b | Nilayer c | Cu d | Ni e | Ni:CuEDX |
|---|---|---|---|---|---|---|---|
| Cus | 1.9 | 20.1 | - | 0.0 | 27.9 | 0 | 0:1 |
| NiCu14s | 5.7 | 14.1 | 0.7 | 4.1 | 25.3 | 14 | 0.65:4 |
| NiCu11s | 14.9 | 20.4 | 0.9 | 12.7 | 13.4 | 49 | 0.96:1 |
| NiCu41s | 21.2 | 20.2 | 0.9 | 18.9 | 6.6 | 74 | 2.8:1 |
| Nis | 14.5 | 34.2 | 0.7 | 10.6 | 0.2 | 98 | 1:0 |
| NiO (Bunsenite) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Lattice Parameter a (nm) | Crystallite Size* (nm) | Sample | Lattice Parameter a (nm) | Crystallite Size * (nm) | ||
| NiCu14g | 0.4186 | 12.1 | NiCu14s | n.d. | n.d. | ||
| NiCu11g | 0.4183 | 13.1 | NiCu11 s | 0.4199 | 13.7 | ||
| NiCu41g | 0.4183 | 18.5 | NiCu41 s | 0.4185 | 11.7 | ||
| Nig | 0.4179 | 33.1 | Ni s | 0.4173 | 9.0 | ||
| CuO (tenorite) | |||||||
| Lattice parameters (nm) | Crystallite size (nm) * | ||||||
| Sample | a | b | c | β (°) | |||
| Cu g | 0.4685 | 0.3427 | 0.5136 | 99.472 | 33.9 | ||
| NiCu14 g | 0.4683 | 0.3427 | 0.5133 | 99.426 | 19.1 | ||
| NiCu11g | 0.4688 | 0.3433 | 0.5124 | 99.330 | 15.8 | ||
| NiCu41g | 0.4690 | 03438 | 0.5120 | 99.270 | 19.7 | ||
| Cus | 0.4730 | 0.3431 | 0.5110 | 99.452 | 27.7 | ||
| NiCu14s | 0.4690 | 0.3488 | 0.5153 | 99.326 | 18.3 | ||
| NiCu11s | 0.4684 | 0.3422 | 0.5107 | 99.231 | 11.7 | ||
| NiCu41s | 0.4686 | 0.3438 | 0.5105 | 99.333 | 19.1 | ||
| Sample | Tonset, °C | mmol H2 goxides−1 | SBET, m2 goxides−1 | Vtot, mm3g−1 | Sample | Tonset, °C | mmol H2 goxides−1 | SBET, m2 goxides−1 | Vtot, mm3g−1 |
|---|---|---|---|---|---|---|---|---|---|
| Cug | 172 | 13.6 | 7 | 15 | Cus | 114 | 18.8 | 9.4 | - |
| NiCu14g | 140 | 13.4 | 22 | 71 | NiCu14s | 155 | 13.1 | 3.3 | - |
| NiCu11g | 140 | 13.5 | 28 | 180 | NiCu11s | 205 | 16.9 | 10.1 | - |
| NiCu41g | 138 | 13.3 | 33 | 116 | NiCu41s | 226 | 17.1 | 10.2 | - |
| Nig | 234 | 14.1 | 14 | 80 | Nis | 342 | 11.8 | 17.8 | - |
| Sample | C 1s | O 1s | Na 1s | Ni 2p | Cu 2p | Ni/ (Ni + Cu)XPS | Ni/ (Ni + Cu)bulk | XPS/ bulk |
| Cug | 54.52 | 32.69 | 2.75 | 0.1 | 9.94 | 0.01 | - | - |
| NiCu14g | 69.72 | 25.93 | 0.33 | 1.74 | 2.28 | 0.43 | 0.21 | 2.0 |
| NiCu11g | 40.2 | 35.23 | 1.31 | 16.79 | 6.47 | 0.72 | 0.53 | 1.4 |
| NiCu41g | 45.55 | 31.84 | 0.31 | 15.82 | 6.47 | 0.71 | 0.80 | 0.9 |
| Nig | 37.82 | 36.4 | 2.05 | 23.72 | 0 | 1 | - | - |
| Sample | C 1s | O 1s | Na 1s | Ni 2p | Cu 2p | Ni/ (Ni + Cu)XPS | Ni/ (Ni + Cu)EDX | XPS/ EDX |
| Cus | 69.2 | 25.8 | - | 0 | 5.0 | 0 | - | - |
| NiCu14s | 62.2 | 28.2 | - | 4.1 | 5.4 | 0.43 | 0.74 | 0.6 |
| NiCu11s | 57.7 | 29.8 | - | 7.2 | 5.2 | 0.58 | 0.49 | 1.2 |
| NiCu41s | 56.5 | 30.0 | - | 8.2 | 5.3 | 0.60 | 0.14 | 4.3 |
| Nis | 69.9 | 26.8 | - | 3.3 | 0 | 1 | - | - |
| Sample | Cug | NiCu14g | NiCu11g | NiCu41g | Nig |
| eV | |||||
| 529.6 | 25.4 | 18.6 | 45.4 | 49.0 | 51.8 |
| 531.9 | 68.6 | 51.8 | 36.6 | 41.3 | 20.7 |
| 533.3 | 6.0 | 29.6 | 18.0 | 9.7 | 27.4 |
| Sample | Cus | NiCu14s | NiCu11s | NiCu41s | Nis |
| eV | |||||
| 529.6 | 16.31 | 30.60 | 43.04 | 43.27 | 11.08 |
| 531.7 | 73.35 | 65.32 | 56.96 | 56.73 | 80.95 |
| 533.8 | 10.34 | 4.08 | 7.98 |
| Catalyst | Granular | Sputtered | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Toluene | Ethanol | Toluene | ||||||||
| T50 | T90CO2 | R130 | T50 | T90CO2 | R270 | T50 | R250 | T90CO2 | T50 | R350 | |
| Cu | 170 | 229 | 0.10 | 281 | 300 | 0.24 | 263 | 78 | 425 | 425 | 26 |
| NiCu14 | 128 | 212 | 0.41 | 272 | 289 | 0.29 | 252 | 98 | 396 | 396 | 32 |
| NiCu11 | 131 | 213 | 0.35 | 273 | 284 | 0.26 | 247 | 148 | 398 | 380 | 36 |
| NiCu41 | 126 | 212 | 0.38 | 274 | 285 | 0.24 | 258 | 93 | 399 | 398 | 21 |
| Ni | 148 | 227 | 0.23 | 297 | 308 | 0.08 | 280 | 85 | 420 | 399 | 18 |
| Parameter | Reactant | Catalyst | ||
|---|---|---|---|---|
| Granular | Sputtered | Pelletized | ||
| T50, °C | Ethanol | 131 | 247 | 160 |
| Toluene | 273 | 380 | 266 | |
| R130 | Ethanol | 0.35 | - | 0.31 |
| R200 | - | 6.02 | 0.87 | |
| R270 | Toluene | 0.26 | - | 0.36 |
| R350 | - | 36.3 | 0.60 | |
| Sample | Nozzle Material | Time, min | Deposition, mg |
|---|---|---|---|
| Cus | Cu | 12; 5; 12 | 1.65 |
| NiCu14s | Cu + Ni (80:20) | 14; 6; 14 | 1.62 |
| NiCu11s | Cu + Ni (50:50) | 18; 8; 18 | 1.30 |
| NiCu41s | Cu + Ni (20:80) | 21; 8; 21 | 1.27 |
| Nis | Ni | 24;10; 24 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirátová, K.; Soukal, P.; Kapran, A.; Babii, T.; Balabánová, J.; Koštejn, M.; Čada, M.; Maixner, J.; Topka, P.; Hubička, Z.; et al. Nickel-Copper Oxide Catalysts Deposited on Stainless Steel Meshes by Plasma Jet Sputtering: Comparison with Granular Analogues and Synergistic Effect in VOC Oxidation. Catalysts 2023, 13, 595. https://doi.org/10.3390/catal13030595
Jirátová K, Soukal P, Kapran A, Babii T, Balabánová J, Koštejn M, Čada M, Maixner J, Topka P, Hubička Z, et al. Nickel-Copper Oxide Catalysts Deposited on Stainless Steel Meshes by Plasma Jet Sputtering: Comparison with Granular Analogues and Synergistic Effect in VOC Oxidation. Catalysts. 2023; 13(3):595. https://doi.org/10.3390/catal13030595
Chicago/Turabian StyleJirátová, Květa, Petr Soukal, Anna Kapran, Timur Babii, Jana Balabánová, Martin Koštejn, Martin Čada, Jaroslav Maixner, Pavel Topka, Zdeněk Hubička, and et al. 2023. "Nickel-Copper Oxide Catalysts Deposited on Stainless Steel Meshes by Plasma Jet Sputtering: Comparison with Granular Analogues and Synergistic Effect in VOC Oxidation" Catalysts 13, no. 3: 595. https://doi.org/10.3390/catal13030595