HPMA Copolymer Mebendazole Conjugate Allows Systemic Administration and Possesses Antitumour Activity In Vivo
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of HPMA Copolymer Conjugate Bearing MBZ
2.2. Cell Lines
2.3. Mice
2.4. IL-2/S4B6 Complexes
2.5. In Vitro Proliferation Assay
2.6. In Vitro Cytotoxicity Assay
2.7. Inhibition of Tumour Growth In Vivo
3. Results and Discussion
3.1. Cytostatic Activity of P-MBZ Conjugates in Cancer Cell Lines of Various Origins In Vitro
3.2. Kinetics of Cytostatic and Cytotoxic Activities of the P-MBZ-A Conjugate and MBZ In Vitro
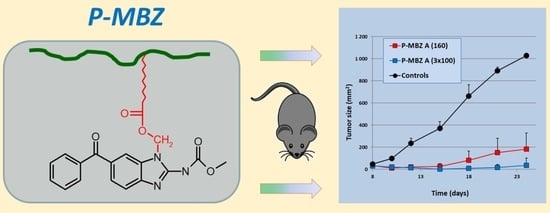
3.3. Antitumour Activity of P-MBZ-A Conjugate In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasaki, J.; Ramesh, R.; Chada, S.; Gomyo, Y.; Roth, J.A.; Mukhopadhyay, T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol. Cancer Ther. 2002, 1, 1201–1209. [Google Scholar]
- Mukhopadhyay, T.; Sasaki, J.; Ramesh, R.; Roth, J.A. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 2002, 8, 2963–2969. [Google Scholar] [PubMed]
- Chu, S.W.; Badar, S.; Morris, D.L.; Pourgholami, M.H. Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole. Anticancer Res. 2009, 29, 3791–3796. [Google Scholar]
- Dogra, N.; Kumar, A.; Mukhopadhyay, T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci. Rep. 2018, 8, 11926. [Google Scholar] [CrossRef]
- Hou, Z.J.; Luo, X.; Zhang, W.; Peng, F.; Cui, B.; Wu, S.J.; Zheng, F.M.; Xu, J.; Xu, L.Z.; Long, Z.J.; et al. Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells. Oncotarget 2015, 6, 6326–6340. [Google Scholar] [CrossRef] [Green Version]
- Jornet, D.; Bosca, F.; Andreu, J.M.; Domingo, L.R.; Tormos, R.; Miranda, M.A. Analysis of mebendazole binding to its target biomolecule by laser flash photolysis. J. Photochem. Photobiol. B 2016, 155, 1–6. [Google Scholar] [CrossRef]
- Doudican, N.; Rodriguez, A.; Osman, I.; Orlow, S.J. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol. Cancer Res. 2008, 6, 1308–1315. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, L.K.; Hewit, K.; Munnings-Tomes, S.; Somani, S.; James, D.; Shanks, E.; Dufes, C.; Straube, A.; Patel, R.; Leung, H.Y. Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment. Br. J. Cancer 2020, 122, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Poruchynsky, M.S.; Komlodi-Pasztor, E.; Trostel, S.; Wilkerson, J.; Regairaz, M.; Pommier, Y.; Zhang, X.; Maity, T.K.; Robey, R.; Burotto, M.; et al. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 1571–1576. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.C.; Moreira Soares, B.; Viana Pinheiro, J.J.; Riggins, G.J.; Pimentel Assumpcao, P.; Rodriguez Burbano, R.M.; Carvalho Montenegro, R. The anthelmintic drug mebendazole inhibits growth, migration and invasion in a gastric cancer cell model. Toxicol. Vitro 2015, 29, 2038–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, L.C.; Moreira-Nunes, C.F.A.; Soares, B.M.; Burbano, R.M.R.; Lemos, J.A.R.; Montenegro, R.C. Mebendazole, an antiparasitic drug, inhibits drug transporters expression in a preclinical model of gastric peritoneal carcinomatosis. Toxicol. Vitro 2017, 43, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.J.; Kim, H.K.; Hong, Y.K.; Joe, Y.A. Autophagy is a potential target for enhancing the anti-angiogenic effect of mebendazole in endothelial cells. Biomol. Ther. 2019, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.Y.; Staedke, V.; Aprhys, C.M.; Gallia, G.L.; Riggins, G.J. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro. Oncol. 2011, 13, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Simbulan-Rosenthal, C.M.; Dakshanamurthy, S.; Gaur, A.; Chen, Y.S.; Fang, H.B.; Abdussamad, M.; Zhou, H.; Zapas, J.; Calvert, V.; Petricoin, E.F.; et al. The repurposed anthelmintic mebendazole in combination with trametinib suppresses refractory NRASQ61K melanoma. Oncotarget 2017, 8, 12576–12595. [Google Scholar] [CrossRef] [Green Version]
- Blom, K.; Senkowsky, W.; Jarvius, M.; Berglund, M.; Rubin, J.; Lenhammar, L.; Parrow, V.; Andersson, C.; Loskog, A.; Fryknas, M.; et al. The anticancer effect of mebendazole may be due to M1 monocyte/macrophage activation via ERK1/2 and TLR8-dependent inflammasome activation. Immunopharmacol. Immunotoxicol. 2017, 39, 199–210. [Google Scholar] [CrossRef]
- Blom, K.; Rubin, J.; Berglund, M.; Jarvius, M.; Lenhammar, L.; Parrow, V.; Andersson, C.; Loskog, A.; Fryknas, M.; Nygren, P.; et al. Mebendazole-induced M1 polarisation of THP-1 macrophages may involve DYRK1B inhibition. BMC Res. Notes 2019, 12, 234. [Google Scholar] [CrossRef]
- Williamson, T.; Bai, R.Y.; Staedtke, V.; Huso, D.; Riggins, G.J. Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumour initiation in a colon cancer preclinical model. Oncotarget 2016, 7, 68571–68584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Li, Y.; Zhang, H.; Huang, E.; Gao, L.; Luo, W.; Wei, Q.; Fan, J.; Song, D.; Liao, J.; et al. Anthelmintic mebendazole enhances cisplatin’s effect on suppressing cell proliferation and promotes differentiation of head and neck squamous cell carcinoma (HNSCC). Oncotarget 2017, 8, 12968–12982. [Google Scholar] [CrossRef]
- Dawson, M.; Braithwaite, P.A.; Roberts, M.S.; Watson, T.R. The pharmacokinetics and bioavailability of a tracer dose of [3H]-mebendazole in man. Br. J. Clin. Pharmacol. 1985, 19, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Dawson, M.; Allan, R.J.; Watson, T.R. The pharmacokinetics and bioavailability of mebendazole in man: A pilot study using [3H]-mebendazole. Br. J. Clin. Pharmacol. 1982, 14, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Kopeček, J. Polymer-drug conjugates: Origins, progress to date and future directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef]
- Tavares, M.R.; Hrabankova, K.; Konefal, R.; Kana, M.; Rihova, B.; Etrych, T.; Sirova, M.; Chytil, P. HPMA-based copolymers carrying STAT3 inhibitor cucurbitacin-D as stimulus-sensitive nanomedicines for oncotherapy. Pharmaceutics 2021, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Pola, R.; Pokorna, E.; Vockova, P.; Bohmova, E.; Pechar, M.; Karolova, J.; Pankrac, J.; Sefc, L.; Helman, K.; Trneny, M.; et al. Cytarabine nanotherapeutics with increased stability and enhanced lymphoma uptake for tailored highly effective therapy of mantle cell lymphoma. Acta Biomater. 2021, 119, 349–359. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Development of HPMA copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv. Drug Deliv. Rev. 2009, 61, 1131–1148. [Google Scholar] [CrossRef]
- Studenovsky, M.; Rumlerova, A.; Kostka, L.; Etrych, T. HPMA-based polymer conjugates for repurposed drug mebendazole and other imidazole-based therapeutics. Polymers 2021, 13, 2530. [Google Scholar] [CrossRef] [PubMed]
- Etrych, T.; Šubr, V.; Strohalm, J.; Šírová, M.; Říhová, B.; Ulbrich, K. HPMA copolymer-doxorubicin conjugates: The effects of molecular weight and architecture on biodistribution and in vivo activity. J. Control. Release 2012, 164, 346. [Google Scholar] [CrossRef]
- Simplício, A.L.; Clancy, J.M.; Gilmer, J.F. Prodrugs for amines. Molecules 2008, 13, 519–547. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Etrych, T.; Chytil, P.; Jelínková, M.; Říhová, B. HPMA copolymers with pH-controlled release of doxorubicin: In vitro cytotoxicity and in vivo antitumor activity. J. Control. Release 2003, 87, 33–47. [Google Scholar] [CrossRef]
- Tomala, J.; Chmelova, H.; Mrkvan, T.; Rihova, B.; Kovar, M. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as a novel approach of cancer immunotherapy. J. Immunol. 2009, 183, 4904–4949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivák, L.; Šubr, V.; Kovářová, J.; Dvořáková, B.; Šírová, M.; Říhová, B.; Randárová, E.; Kraus, M.; Tomala, J.; Studenovský, M.; et al. Polymer-ritonavir derivate nanomedicine with pH-sensitive activation possesses potent anti-tumor activity in vivo via inhibition of proteasome and STAT3 signaling. J. Control. Release 2021, 332, 563–580. [Google Scholar] [CrossRef] [PubMed]








| Conjugate | Mw kDa | Ɖ | Size (Dh) nm | TT/MBZ Content mmol⋅g−1 | Polymerisation Technique |
|---|---|---|---|---|---|
| poly(HPMA-co-Ma-β-Ala-TT) (P-MBZ-A precursor) | 28 | 1.4 | 8.2 | 0.33 | free radical |
| poly(HPMA-co-Ma-β-Ala-TT) (P-MBZ-B precursor) | 35 | 1.1 | 8.6 | 0.35 | RAFT |
| P-MBZ-A | 32 | 1.6 | 10.1 | 0.28 | free radical |
| P-MBZ-B | 37 | 1.1 | 10.3 | 0.22 | RAFT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studenovský, M.; Rumlerová, A.; Kovářová, J.; Dvořáková, B.; Sivák, L.; Kostka, L.; Berdár, D.; Etrych, T.; Kovář, M. HPMA Copolymer Mebendazole Conjugate Allows Systemic Administration and Possesses Antitumour Activity In Vivo. Pharmaceutics 2022, 14, 1201. https://doi.org/10.3390/pharmaceutics14061201
Studenovský M, Rumlerová A, Kovářová J, Dvořáková B, Sivák L, Kostka L, Berdár D, Etrych T, Kovář M. HPMA Copolymer Mebendazole Conjugate Allows Systemic Administration and Possesses Antitumour Activity In Vivo. Pharmaceutics. 2022; 14(6):1201. https://doi.org/10.3390/pharmaceutics14061201
Chicago/Turabian StyleStudenovský, Martin, Anna Rumlerová, Jiřina Kovářová, Barbora Dvořáková, Ladislav Sivák, Libor Kostka, Daniel Berdár, Tomáš Etrych, and Marek Kovář. 2022. "HPMA Copolymer Mebendazole Conjugate Allows Systemic Administration and Possesses Antitumour Activity In Vivo" Pharmaceutics 14, no. 6: 1201. https://doi.org/10.3390/pharmaceutics14061201