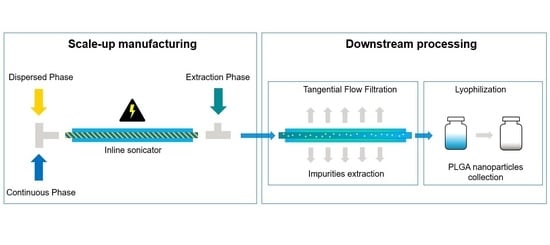
The inline indirect sonication method was further investigated for PLGA-based nanomedicine scale-up manufacturing. First, the organic solvent used to prepare the polymer solution was switched from DCM to EtOAc due to the lower toxicity and higher water solubility of EtOAc compared to DCM. In fact, in accordance with the ICH guidelines [
36], DCM is a class 2 solvent with a permitted daily exposure (PDE) equivalent to 6.0 mg/day. Conversely, EtOAc lies in class 3, meaning that the PDE corresponds to 50 mg/day [
36]. Therefore, working with large amount of a less toxic solvent increases the safety of employees at work while reducing the complexity of safety protocols that must be prepared and heeded. Also, the higher degree of water solubility of EtOAc (8 g/100 mL versus 1.3 g/100 mL for DCM at 25 °C [
30,
37]) would require smaller volumes of water as the extraction phase and render the process easier to handle. Although therefore many substances should be reduced or directly replaced with greener ones, it is not always possible to choose the desired solvents because not all substances have the same solubility in various solvents. For example, super critical CO
2 offers the advantages of being non-toxic and leaving no residue. On the other hand, the limited choice of soluble materials and the compatibility of organic solvents with the technical apparatus hinder its industrial scalability [
1,
38]. Therefore, in this case, during scale-up experiments, EtOAc was chosen over DCM because of its lower toxicity and unaltered ability to solubilize PLGA, while DMSO was selected for its ability to dissolve a wide variety of active ingredients. Thus, the DMSO/EtOAc mix was identified as optimal for most formulations to be manufactured. Nonetheless, it is important to note that, although notoriously toxic, the use of DCM in particle production is still widespread and has been reported for the production of formulations used in phase I clinical trials [
39]. Keeping in mind the required specification of 150 ± 50 nm and PDI < 0.2, a set of particles was generated via indirect inline sonication technique with the scope of scaling-up the technology. The experiment parameters are reported in
Table 3, exp. 3–7. The influence of polymer concentration and the TFR on the particle size and PDI were studied systematically.
Figure 7A shows the variations of particle size as a function of PLGA concentration (5 wt%, 10 wt% and 20 wt%) obtained at a TFR of 8 mL/min. Given the greater water solubility of EtOAc compared to DCM, an extraction phase ratio of 3 (3 folds the amount of water required to solubilize EtOAc) was adopted, bringing the EP flow rate at 60 mL/min. This was done in order to dilute the particle suspension enough to be processed on the same day at the TFF, avoiding potential damage of the hollow fiber filter caused by the solvent. As expected, the use of EtOAc resulted in smaller particles than DCM due to the lower interfacial tension of the solvent with water, as previously observed in the case of DMSO addition. Also, as already experienced in one of our previous works [
6], a gradual increase in particle size and PDI was noticed for higher concentrations of PLGA. While 5 wt% polymer resulted in particles with a mean diameter of approx. 85 nm, 125 nm particles were obtained with 20 wt% of polymer concentration with a PDI of approx. 0.1 for all formulations. As 10 wt% and 20 wt% PLGA were in the specification range, the higher PLGA concentration in EtOAc was chosen as the polymer concentration for the subsequent experiments due to the higher throughput of 19 g/h obtained with this polymer concentration. The influence of the TFR on the particle size and PDI is shown in
Figure 7B. Accordingly, an increase in particle size was observed at higher TFR. This is due to shorter sonication time for the faster-flowing samples, leading to less homogenizing treatment time. 32 mL/min was found to result in particles still in the target size, therefore, it was chosen for the production of the loaded particles given the high throughput obtained from the high continuous flow (approx. 76 g/h).
As a comparison, the batch production method was also implemented in large scale (
Table 4, exp. 10). Briefly, 20 wt%
w/w PLGA in EtOAc was mixed with DMSO and sonicated for 0.5 min with PVA 2 wt%. Subsequently, the homogenized suspension was transferred to 81 mL of water used as the extraction phase.
Figure 7C shows the particle size produced by this method. As it can be noted, the particle size as well as the PDI are out of the required specifications, and the values are considerably higher than those obtained with the inline method. This shows that inline sonication is easier to scale up and that, to allow for increased batch scale, a new process with a different probe would have to be entirely reevaluated.
Ritonavir and Celecoxib Nanoparticles
To further confirm the usefulness of the inline production method, ritonavir and celecoxib were chosen as model drugs and were encapsulated within PLGA nanoparticles. Ritonavir is an antiretroviral protease inhibitor API that is widely used in combination with other medications for the treatment of human immunodeficiency virus (HIV) infection, which causes the acquired immunodeficiency syndrome (AIDS) [
13]. Celecoxib is a cyclo-oxygenase-2 (COX-2) selective inhibitor used in the treatment of pain and inflammation [
14]. It is one of the most commonly prescribed COX-2 specific inhibitors since its use effectively reduces clinical gastrointestinal events in comparison to other nonsteroidal anti-inflammatory drugs (NSAIDs). Both of these APIs have already been investigated regarding their ability to be entrapped into PLGA nanoparticles in order to overcome problems such as low patient therapy adherence [
13] and important side effects [
14].
Since ritonavir and celecoxib are hydrophobic compounds, PLGA and API are dissolved together in the organic phase and then emulsified with the aqueous solution containing the surfactant. Otherwise, encapsulation of a hydrophilic API would require an initial formation of a water-in-oil emulsion, in which the API is in the aqueous phase and the polymer is in the organic phase. Next, the water-in-oil emulsion is mixed with a second aqueous solution containing the surfactant, creating a water-in-oil-in-water system [
1]. The experimental conditions for large-scale manufacturing of ritonavir and celecoxib nanoparticles are shown in
Table 3 (exp. 8–9). Both the nanoparticle types were produced achieving a total yield of 84 g/h, which corresponds to approx. 2 kg/day when run continuously. The characteristics of the nanoparticles are shown in
Figure 8. The particle size achieved with this method was below 200 nm for both formulations. Ritonavir encapsulation led to particles of 188.5 ± 10.2 nm and PDI of 0.19 ± 0.09. Zeta potential was registered at −37.4 ± 3.0 mV, meaning that the particles were overall stable. Celecoxib resulted in particles of similar size and PDI of 184.7 ± 1.2 nm and 0.17 ± 0.01. Zeta potential was slightly more negative, being −42.8 ± 2.5 mV. Encapsulation efficiency of both the APIs was determined with HPLC. As shown in
Table 8, the efficiency of ritonavir and celecoxib encapsulation was approx. 50% and 80%, respectively. Although ritonavir and celecoxib may possess some similar characteristics such as no formal charge and very poor water solubility, their functional molecular groups as well as molecular weights are substantially different [
40,
41]. Therefore, it is reasonable that each difference may decree a variation in the interaction of the API with the polymer matrix, resulting in a unique encapsulation capacity.
Table 9 summarizes some of the most prominent physicochemical characteristics of the APIs.