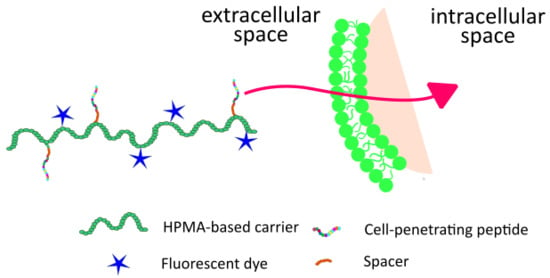
1. Introduction
Cell-penetrating peptides (CPPs) are short oligopeptides that were designed and described as moieties that are able to readily penetrate into living cells. Most commonly, they are used for delivery of certain cargoes into the cells. The cargo is usually a hydrophobic or a large molecule that does not easily get across the cell membrane alone. The number of CPPs described in literature is relatively high and it is still rising. The efficiency of the penetration depends on the particular structure of the CPP, as well as on the cell type, temperature, concentration, and duration of the particular experiment. According to the structure, CPPs may be categorized into the three main groups—positively charged; hydrophobic, and amphiphilic peptides [
1]. The CPP can be directly attached to the cargo by a covalent bond [
2] or both the CPP and the cargo can be attached together to suitable carriers [
3] such as metal nanoparticles [
4], polymers [
5], or liposomes [
6]. The detailed structure of each system can substantially affect the cell penetration process.
Recently, various water-soluble and micellar polymer drug carriers have been developed and studied in detail; among them
N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers have a significant position. These materials are well-defined copolymers perfectly tolerated in the human body; they are water-soluble, non-immunogenic, biocompatible, and clearable from organism by renal filtration (up to molecular weight ~70,000 g·mol
−1) [
7]. The indisputable advantage of this polymer system is its hydrophilicity, enabling solubilization of various hydrophobic drugs after their attachment to the polymer [
8]. Conjugation of the copolymer with either a fluorescent label or a radionuclide provides powerful polymer diagnostic probes for detection of malignant tumors [
9]. It was discovered that HPMA-based copolymers (as well as other macromolecules) are preferentially accumulated in tumor tissue due to the leaky and permeable tumor vascular system. This phenomenon is known as the so-called Enhanced Permeability and Retention (EPR) effect [
10]. Preferably, the EPR effect in combination with efficient CPPs bound to the polymer chain could enhance internalization of some anti-tumor drugs into the malignant cells [
11,
12]. In some therapeutic applications, the internalization of the drug into the cancer cells is a prerequisite to its activity. e.g., in photodynamic therapy, the development of singlet oxygen inside the target cell is a crucial parameter for the high efficacy of tumor cell destruction [
13]; therefore, a polymer carrier bearing both CPP and a photosensitizer could substantially improve the outcome of the therapy.
In this work, an HPMA-based copolymer was used as a carrier of both a fluorescent label and a CPP as a cell uptake enhancer. The presented system can be used either directly as a diagnostic probe for tumor imaging or even for fluorescence-guided surgery; eventually, upon replacement of the fluorophore by an anticancer drug it might be utilized as a polymer therapeutic system with enhanced accumulation in solid tumors and with improved penetration into the cancer cells.
For our comparative study we have chosen six different cell-penetrating peptides that can be divided into the following categories: (1) three positively charged peptides—the historically first described cell-penetrating peptide GRKKRRQRRR (TAT) [
14,
15] derived from the transactivator of transcription protein of the human immunodeficiency virus, minimal sequence of penetratin RRMKWKK (PEN) derived from third α-helix of the Antennapedia-based homeoprotein first discovered in
Drosophila [
16,
17], and the peptide RYIRS (RY) [
18]; (2) one amphipathic peptide—YARAAARQARA (YA) derived from TAT and called PTD4 [
19]; and (3) two short hydrophobic peptides—VPMLK (VP) [
20] and PFVYLI (PF) [
21].
The main aim of the current work was to investigate to what extent these CPPs could enhance internalization of the labeled polymer conjugates into the cancer cells at a wide range of concentrations. Moreover, another goal was to select the most efficient CPPs and to study how the length of the spacer between the polymer carrier and CPP can influence the penetration efficacy of the system. Finally, the focus on cell internalization and the cytotoxic effect of the selected conjugates decorated with cytostatic drug pirarubicin is described.
2. Materials and Methods
2.1. Chemicals
Methacryloyl chloride (MA-Cl), 2-thiazoline-2-thiol (TT), 2,2′-azobis(isobutyronitrile) (AIBN), 1-hydroxybenzotriazole (HOBt), tert-butyl alcohol (t-BuOH), N,N-dimethylacetamide (DMA), triisopropylsilane (TIPS), 3-mercaptopropionic acid (SH-acid), N,N′-diisopropylcarbodiimide (DIC), 2-cyano-2-propyl benzodithioate (DTB-AIBN), and o-phthalaldehyde (OPA) were purchased from Sigma-Aldrich, Prague, Czech Republic. N-Ethyldiisopropylamine (DIPEA), N,N-dimethylformamide (DMF), ethyl cyanoglyoxylate-2-oxime (Oxyma), Tenta Gel R RAM resin, 9-fluorenylmethoxycarbonyl-amino acids (Fmoc-AA), piperidine (Pip), (benzotriazol-1-yloxy) trispyrrolidinophosphonium hexafluorophosphate (PyBOP), trifluoroacetic acid (TFA), 1-(9-fluorenylmethyloxycarbonyl)amino-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-oic acid (Fmoc-Peg12-COOH), and 15-(9-fluorenylmethyloxycarbonyl)amino-4,7,10,13-tetraoxa-pentadecanoic acid (Fmoc-Peg4-COOH) were purchased from Iris Biotech, GmbH, Marktredwitz, Germany. Dyomics 633 amino derivative (Dy633) was purchased from Dyomics GmbH, Jena, Germany. 1-Aminopropan-2-ol (AMP) was purchased from Tokyo Chemical Industry Co., Ltd., Tokyo, Japan. Initiator 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (V70) was purchased from FUJIFILM Wako Pure Chemical Corporation, Neuss, Germany. 5-Azidopentanoic acid (N3-pent-COOH) was purchased from Bachem, Bubendorf, Switzerland. Amino-1-(11,12-didehydrodibenzo[b,f]azocin-5(6H)-yl) propan-1-one (Dbco-NH2) was purchased from Click Chemistry Tools, Scottsdale, AZ, USA. Methanol, acetonitrile, and all other solvents were purchased from VWR International s. r. o., Stříbrná Skalice, Czech Republic. All chemicals and solvents were of analytical grade. The solvents were dried and purified by conventional procedures. All amino acids were l-configuration. Pirarubicin (Pir) was obtained from Meiji Seika Pharma Co., Ltd. (Tokyo, Japan).
2.2. Analytical Methods
Control of peptide purity and monitoring of subsequent coupling reactions with polymer carrier was performed by HPLC (Shimadzu, Kyoto, Japan) using Chromolith Performance RP-18e, 100 × 4.6 mm column (Merck, Darmstadt, Germany). A linear gradient of water-acetonitrile (5–95 vol % of acetonitrile) with the presence of 0.1 vol % TFA was used as a mobile phase and the flow rate was set to 5 mL·min−1. Detection was performed via a UV/VIS photodiode array detector (Shimadzu, Japan) and a fluorescence detector RF-10AXL (Shimadzu, Japan). The content of attached peptides was determined by amino acid analysis. Samples were hydrolyzed (6 M HCl, 115 °C, 16 h in sealed ampule) then modified with OPA and SH acid immediately before injection to HPLC using the same column as was described above and for fluorescence detection (excitation 229 nm, emission 450 nm) and gradient elution (10–100% of solvent B in 35 min, flow rate 1.0 mL·min−1) with buffers of composition as follows: solvent A: 0.05 M sodium acetate buffer, pH 6.5; solvent B: 300 mL of 0.17 M sodium acetate and 700 mL of methanol. Molecular weight and dispersity were determined by size exclusion chromatography (SEC) using HPLC (Shimadzu) equipped with a TSK 3000 SWXL column (Tosoh Bioscience, Tokyo, Japan) and refractive index (RI) and UV (Shimadzu), differential viscometer ViscoStar III, and multiangle light scattering DAWN 8 EOS (Wyatt Technology Corp., Santa Barbara, CA, USA) detectors. Measurements were performed using a mobile phase composed of 80 vol % methanol and 20 vol % 0.3 M acetate buffer (pH 6.5) at a flow rate of 0.5 mL·min−1. Contents of reactive TT groups (ε305 = 10,300 L·mol−1·cm−1, MeOH), Dy633 (ε633 = 200,000 L·mol−1·cm−1, EtOH), and pirarubicin (ε488 = 11,300 L·mol−1·cm−1, MeOH) were determined spectrophotometrically using a SPECORD 205 Spectrometer (Analytik Jena AG, Jena, Germany). Matrix-assisted laser desorption/ionization time of flight mass spectroscopy (MALDI-TOF MS) was performed on a Bruker Biflex III mass spectrometer. The molecular mass of the peptide products was determined using mass spectrometry performed on an LCQ Fleet mass analyzer with electrospray ionization (ESI MS) (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
2.3. Synthesis of Monomers
N-(2-hydroxypropyl)methacrylamide (HPMA) was prepared by the reaction of methacryloyl chloride with 1-aminopropan-2-ol in dichloromethane as described earlier [
22]. 3-Methacrylamidopropanoic acid (Ma-β-Ala-OH) was synthesized by the reaction of methacryloyl chloride with 3-aminopropanoic acid in aqueous alkaline medium [
23]. 3-(3-Methacrylamidopropanoyl)thiazolidine-2-thione (Ma-β-Ala-TT) was prepared by the reaction of Ma-β-Ala-OH with 4,5-dihydrothiazole-2-thiol in the presence of 4-dimethylaminopyridine [
23]. The monomer was characterized using HPLC and ESI MS (calculated 258.3, found 259.1, M + H).
2.4. Synthesis of the Polymer Precursor Poly(HPMA-co-Ma-β-Ala-TT)
The title copolymer with reactive TT groups was prepared by reversible addition-fragmentation chain-transfer (RAFT) polymerization of HPMA (1.5 g, 10.47 mmol) and Ma-β-Ala-TT (369 mg, 1.42 mmol) in a mixture of 85 vol % of
t-BuOH and 15 vol % of DMA. Reaction was carried out in a sealed ampule under argon at 40 °C for 16 h. DTB-AIBN (13.17 mg) was used as the chain-transfer agent (CTA) and V-70 (9.17 mg) was used as initiator (INI) [
23]. The resulting polymer solution was precipitated into a mixture of acetone and diethylether (1:1). Polymer was then filtered and dried in a vacuum. The polymer was dissolved in DMA (1.5 mL) with 20 wt % of AIBN and heated to 80 °C for 2 h to remove the terminal DTB groups [
24]. The final polymer precursor
1 was then analyzed by SEC; molecular weight was
= 27,500 g∙mol
−1 with polydispersity index
Ð = 1.03. Analysis using UV/VIS spectrophotometer showed content of TT groups equal to 11.6 mol %.
2.5. Synthesis of Peptides
Peptides were synthesized using a standard Fmoc strategy using a Liberty Blue microwave peptide synthesizer (CEM, Matthews, NC, USA) and Tenta Gel R ring amide resin. Starting from the C-terminus the synthesis was performed automatically with 2.5 equivalent of the N-Fmoc-protected amino acid derivative, 2.5 equivalent of DIC as an activator, and 2.5 equivalent of Oxyma as an activator base in DMF in each step. After microwave coupling and washing, Fmoc protecting group was cleaved. Part of the final peptide was than modified with Fmoc-Peg12-COOH or Fmoc-Peg4-COOH, respectively. In the last coupling step, N3-pent-COOH was attached to the N terminus. Finally, the peptides were cleaved from the resin using a mixture of 95 vol % TFA, 2.5 vol % TIPS, and 2.5 vol % water for 3 h. The resin was removed by filtration, the filtrate was concentrated under reduced pressure, and the crude peptide was isolated by precipitation with cold diethyl ether followed by filtration. Purity and identity of the prepared peptides was determined by HPLC and MALDI-TOF MS.
2.6. Synthesis of Enzymatically Cleavable Pirarubicin Derivative—N3-pent-GFLG-Pir
N
3-pent-GFLG-OH was prepared on 2-chlorotrityl chloride resin as described earlier [
23]. Pirarubicin was attached to the peptide carboxylic group using DIC/NHS activation similarly as described in our previous work [
23], except that the condensation of the peptide with pirarubicin was performed without DIPEA. Finally, the product was purified using column chromatography (silicagel, chloroform, and methanol 95:5). The yield was 27.5 mg (0.0244 mmol, 51%) of the title compound. ESI MS (calculated 1126.5, found 1149.5 M + Na).
2.7. Synthesis of the Polymer Conjugates
Fluorescently labeled polymer–peptide conjugates were synthesized via a three-step procedure. First, polymer precursor
1 (120 mg, 91.7 μmol TT) was reacted with Dbco-NH
2 (2 mol %, 4.37 mg, 15.8 μmol) in DMA (1.2 mL) in the presence of DIPEA (2.7 μL) as shown in
Scheme 1. Monitoring of the coupling was done by HPLC. After 1 h, all Dbco-NH
2 was reacted. In the second step, fluorescent dye Dy633-NH
2 (0.5 mol %, 3 mg, 3.89 μmol) dissolved in DMA (100 μL) was added in the presence of DIPEA (0.67 μL) to the reaction mixture. To remove the remaining TT groups, AMP (7 μL, 91.7 μmol) was added to the reaction mixture. The resulting polymer precursor
2 was separated by precipitation to mixture of acetone and diethylether (1:1). The third step of synthesis was copper-free click reaction of azide derivatives of peptide TAT, PEN, YA, RY, VP, and PF (1.5 mol %) with Dbco groups of the polymer. After 1 h, all peptide was attached according to HPLC analysis. Final polymer–peptide conjugates were isolated by precipitation to a mixture of acetone and diethyl ether (1:1) and dried to constant weight. Reference polymer
REF was prepared similarly. Briefly, polymer precursor
1 (20 mg, 15.3 μmol TT) was reacted with Dy633-NH
2 (0.5 mol %, 0.5 mg) in the presence of DIPEA (0.1 μL) in DMA (0.2 mL). Remaining TT groups were removed by addition of AMP (1.2 μL, 15.3 μmol) and polymer was precipitated and dried, monitoring of all reaction was performed by HPLC. All polymers were dissolved in water, purified by chromatography on Sephadex G 25 resin in water (PD 10 column, GE Health care), and freeze-dried. The amount of fluorescent dye was determined spectrophotometrically in methanol (ε
633 = 200,000 L·mol
−1·cm
−1) and amount of bound CPP was determined by amino acid analysis. For preparation of polymer–peptide conjugates with various spacer lengths, polymer precursor
2 was reacted with N
3-pent-TAT/PEN and N
3-pent-Peg
12-TAT/PEN peptides, respectively.
Synthesis of drug bearing polymer–peptide conjugate was performed in three steps. In the first step, polymer precursor 1 (25 mg, 19.1 μmol TT) was reacted with Dbco-NH2 (6 mol %, 2.63 mg, 9.5 μmol) in DMA (0.3 mL) in the presence of DIPEA (1.6 μL). Remaining TT groups were removed by adding AMP (1.55 μL, 20.3 μmol) to the reaction mixture. The resulting polymer precursor was precipitated to a mixture of acetone and diethyl ether (1:1) and dried. The second step of synthesis was 1 h lasting “click” reaction of N3-pent-Peg12-TAT (4 mg, 0.944 μmol) to the Dbco-containing polymer precursor in water (0.5 mL). The reaction mixture was freeze-dried and the solid was dissolved in DMA (0.3 mL). Finally, N3-pent-GFLG-Pir (4.78 mg, 4.24 μmol) was bound to the polymer precursor by “click” reaction. All reaction steps were monitored by HPLC. The final product P-T12-Pir was isolated by precipitation to ethyl acetate. The content of pirarubicin in the polymer conjugate was determined spectrophotometrically in methanol (ε488 = 11,300 L·mol−1·cm−1) and the content of CPP was determined by amino-acid analysis. The conjugate without peptide P-Pir was prepared by the same procedure, only the amount of Dbco-NH2 was reduced (3.5 mol %, 1.54 mg, 5.53 μmol) and the step with the peptide click reaction was excluded. N3-pent-GFLG-Pir (3.98 mg, 3.53 μmol) was bound to polymer precursor as described above.
Characteristics of all prepared polymers are summarized in
Table 1.
2.8. Cell Culture
The HeLa cell line (LGC Standards, Poland) was cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 100 U of penicillin, 100 μg⋅mL−1 streptomycin, and 10% fetal bovine serum in 25 cm2 flasks. Cells were cultivated in a humidified incubator at 37 °C with 5% CO2. The chemicals were purchased from Life Technologies/Gibco, Prague, Czech Republic.
2.9. Flow Cytometry
The HeLa cell line was incubated with fluorescently labeled polymer–peptide conjugates for 1 h at 4 °C and 37 °C. For measurement at 4 °C, HeLa cells were harvested with 0.05% trypsin (Thermo Fisher Scientific, Czech Republic) at 37 °C for 3 min. Subsequently, cells were washed with 0.5% bovine serum albumin in phosphate buffered saline (0.01 M) (BSA/PBS), pre-cooled for 15 min and incubated with fluorescently labeled polymer–peptide conjugates in the dark for 1 h at 4 °C. After incubation, the cells were washed once with BSA/PBS, centrifuged at 1500 rpm for 3 min and re-suspended in 1 μM Sytox Blue Dead Cell Stain (Thermo Fisher Scientific, Czech Republic) to distinguish live and dead cells. For measurement at 37 °C, HeLa cells were seeded 24 h prior to the experiment on 24-well plates at a density 1.5 × 105 cells per well. Polymer–peptide conjugates were then added together with fresh DMEM and incubated at 37 °C in 5% CO2 and humidified atmosphere. Subsequently, cells were washed with BSA/PBS, harvested with 0.05% trypsin at 37 °C for 3 min, transferred to tubes, centrifuged at 1500 rpm for 3 min, and re-suspended in PBS/BSA containing 1 μM Sytox Blue Dead Cell Stain (Thermo Fisher Scientific, Czech Republic). All measurements were acquired using a FACSVerse™ flow cytometer (Becton Dickinson Czechia, s.r.o., Prague, Czech Republic) and analyzed by FlowJo software version 10 (Tree Star Inc., Ashland, OR, USA); the median of fluorescence intensity was determined. The concentration of polymer conjugates was adjusted to the value corresponding to a final concentration of peptide 1000 μM. This stock solution was diluted with PBS to obtain all used concentrations. REF polymer conjugate was used in a final concentration 11.6 mg⋅mL−1, which corresponds to the amount of dye in the corresponding polymer–peptide conjugates. HeLa cells without a polymer were used as a negative control. All samples were measured in duplicates in two independent experiments.
2.10. Laser Scanning Confocal Microscopy
Laser scanning confocal microscopy (LSCM) was used to visualize the intracellular uptake of selected polymer conjugates. HeLa cells were seeded 24 h prior to an experiment (1.5 × 105 cells per well) to confocal chambers (1 μ-dishes providing a 35 mm2 growth area with 500 μL cell culture medium) with four chambers in a 20 mm micro-well and a #1 cover glass (0.13–0.16 mm) (Bio-Port Europe, Prague, Czech Republic). Medium was replaced by fresh medium with dissolved polymer–peptide conjugates and added to confocal chamber and incubated either at 4 °C or at 37 °C for 1 h. After the incubation time, cells were washed twice with PBS and measured by confocal microscopy. Hoechst 33342 was used for visualization of cell nuclei (5 μg·mL−1, Thermo Fischer Scientific, Czech Republic) and cell membranes were stained with CellMask™ Green (1 μg⋅mL−1, Thermo Fisher Scientific, Czech Republic) for 1 h before imaging at 4 °C or 10 min at 37 °C. Observation of samples was performed by Olympus IX83 coupled with FV10-ASW software (Olympus, Prague, Czech Republic). The samples were scanned using the 60× oil objective Plan ApoN (1.42 numerical apertures). Dy633-labeled polymers were excited at 637 nm and emitted light was detected through a 650–750 nm filter. For the detection of Hoechst 33342 dye-stained nuclei, an excitation wavelength of 405 nm was used and emitted light was detected through a 420–500 nm filter. CellMask Green-stained cell membranes were excited at 488 nm and the emitted light was detected through a 500–600 nm filter. All samples were measured in duplicate in two independent experiments.
2.11. Cell Viability
Cytotoxicity of polymer–peptide conjugates was determined using Alamar Blue® cell viability reagent (Thermo Fischer Scientific, Czech Republic) in HeLa cells according to the manufacturer’s protocol. Cells were seeded into 96-well plates in 100 μL of DMEM 24 h prior to experiment at a density of 5 × 103 cells per well. The medium was then replaced by 100 μL of the fresh medium with a serial dilution of TAT4, PEN4, REF, and P-T12-Pir, P-Pir, respectively. Cells were subsequently incubated for 24 h in 5% CO2 at 37 °C. Then the medium was replaced by 90 μL of fresh medium with 10 μL of Alamar Blue® reagent and incubated for the next 4 h in 5% CO2 at 37 °C. Resazurin, the active compound of Alamar Blue® reagent, was reduced to the highly fluorescent compound resorufin only in viable cells. The fluorescence intensity was measured using a Synergy Neo plate reader (Bio-Tek, Prague, Czech Republic) with excitation at 550 nm and emission at 590 nm. Non-treated cells were used as a negative control and free pirarubicin as a positive control. All samples were measured in triplicate in three independent experiments. The half maximal inhibitory concentration value IC50 was expressed as a concentration of CPP ± SD and pirarubicin ± SD, respectively. The cytotoxic response of HeLa cells to the above-mentioned polymer–peptide conjugates was measured in the concentration range from 98 nM to 100 μM of CPP, concentration of polymer conjugate REF was used in a range from 1.1 mg⋅mL−1 to 1.1 μg⋅mL−1. In the case of drug bearing polymer conjugates, the concentration varied from 43.2 nM to 44.3 μM of pirarubicin.
2.12. Statistical Analysis
Results were plotted as average ± standard deviation (SD). All statistical analysis was performed using GraphPad Prism 5.03. An ANOVA test was followed by Dunnett’s or Turkey’s test. A value of *** p < 0.001, ** p < 0.01, and * p < 0.05 was considered statistically significant. All IC50 (the concentrations of the CPP or drug reducing the cell viability to one half) values were obtained from calculations corresponding to fitting by logistic S-curves.
4. Conclusions
The major goal of this study was to investigate the influence of a CPP covalently attached to water-soluble polymer–drug conjugate onto the cellular uptake of the conjugate and consequently also its cytotoxicity. Various CPP and spacers between polymer chain and CPP were studied in detail and compared. Fluorescently labeled polymer conjugates decorated with cell-penetrating peptides often mentioned in literature were successfully prepared using copper-free click chemistry. Cell-penetration ability was compared considering identical molar amount of peptide on each conjugate representing in average three molecules of peptide per one polymer chain. Flow cytometry showed that TAT peptide attached along the polymer chain is rightfully the most favorite and efficient in delivery of cargo across the cell membrane. At lower concentrations, a minimal sequence of penetratin (PEN) also showed decent cell-penetrating ability. This observation was supported also by confocal microscopy experiments.
Concerning the spacer length influence, the longest dodeca(ethylene glycol) spacer proved to have a favorable effect to the cell penetration of the both studied polymer–peptide conjugates compared to the corresponding spacer-free polymer conjugates while the shorter tetra(ethylene glycol) spacer improved only the penetration of the TAT conjugate, but it did not improve the penetration of the PEN conjugate.
After attachment of a cytostatic drug pirarubicin, we could observe significantly increased cytotoxicity of the polymer–drug-CPP conjugate compared with the polymer–drug conjugate without CPP. The improved cytotoxicity effect is apparently caused by TAT-mediated enhanced penetration into HeLa cells.
Efficient internalization of a cytostatic drug into the target malignant cells is often a prerequisite for successful therapy; e.g., for application of DNA intercalators or photosensitizers producing singlet oxygen in photodynamic therapy, the intracellular localization of the drug is an absolute necessity. We believe that the CPP–polymer conjugates with various cytostatic drugs could be used as a new generation of therapeutic agents for a more efficient cancer treatment. Finally, the presented CPP–polymer conjugates with fluorophores could eventually be used as polymer probes for diagnosis or for fluorescently guided surgery of various malignant tumors. The efficacy of the presented TAT- and PEN-decorated polymer conjugate will be evaluated in the oncoming study for its in vivo efficacy with the aim of studying in detail the combination of EPR effect and CPP penetration in tumor models.