The Effects of Copolymer Compatibilizers on the Phase Structure Evolution in Polymer Blends—A Review
Abstract
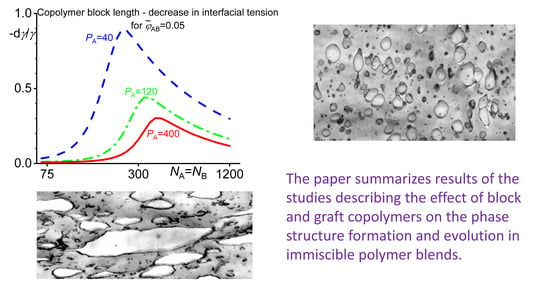
:1. Introduction
2. Phenomenological Predictive Rules
3. Distribution of a Copolymer in Polymer Blends and the Interfacial Tension
3.1. Calculation of the Equilibrium Distribution of a Copolymer between the Interface and Bulk Phases
3.2. Theories Considering the Effect of Finite Fraction of a Copolymer at the Interface on Its Distribution in Blends
3.3. The Effect of a Copolymer Geometry on the Interfacial Tension in Compatibilized Polymer Blends
3.4. The Effect of a Copolymer Micellization on the Interfacial Tension in Compatibilized Polymer Blends
4. The Effect of a Compatibilizer on the Droplet Breakup
4.1. Formulation of the Problem
4.2. Available Results
5. The Effect of a Compatibilizer on the Coalescence
- 1.
- Approaching of the droplets;
- 2.
- Drainage of the continuous phase trapped between the droplets, possibly deformed by the axial force;
- 3.
- Rupture of the rest of continuous phase after the droplet approach to the critical distance, hc, usually by the formation of a “hole” on the thinnest spot;
- 4.
- Evolution of the “neck” to form a coalesced droplet.
6. The Competition between Breakup and Coalescence in Compatibilized Blends
7. Discussion of Some Experimental Results with Respect to the Available Theories
7.1. Emulgation Curves
7.2. The Effect of a Copolymer Molecular Weight on Its Compatibilization Efficiency
7.3. Distribution of a Copolymer in Polymer Blends in Steady Flow and Quiescent State
8. Summary of the Present State of the Art
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a | segment length; |
| c | concentration; |
| Ccrit | critical copolymer concentration according to ref. [5]; |
| Ca | capillary number, see Equation (20); |
| Ds | the surface diffusivity of the copolymer; |
| d | diameter; |
| dmin | minimum average droplet diameter; |
| e | the Euler number |
| f | coefficient relating to a decrease in the interfacial tension for the dry brush system in chapter 4; |
| fA | ratio of A block to the whole copolymer lengths, fA = NA/N.; |
| G | the shear rate; |
| I | unit tensor; |
| jC, jD | the convective and diffusive flux, respectively; |
| K | parameter of proportionality; |
| k | the Boltzmann constant; |
| k | index denoting specific phase (if used in a superscript or subscript); |
| lB | corona thickness; |
| M | auxiliary parameter in Equation (23); |
| N | total segments number in the copolymer chain; |
| Nj | number of segments in the copolymer block j; |
| n | the unit normal directed outward from the droplet; |
| Pi | number of segments of the homopolymer i; |
| p | viscosity ratio of the droplets and matrix, p = ηd/ηm; |
| q | position dependent density; |
| R | the droplet radius; |
| R0 | radius of non-deformed spherical droplet; |
| S | interfacial area in volume unit of a blend; |
| sr | the ratio of swelling powers ratio of block copolymer segments at the interface outside the droplet versus those inside the droplet; |
| sij | swelling power of the homopolymer i in the copolymer block j; |
| T | temperature; |
| Tm and Td | the stress tensors in the matrix and dispersed phase, respectively; |
| t | time; |
| tB | breakup time; |
| αB | stretching of B blocks of a copolymer in micelle corona; |
| Γ | the surface to volume parameter, Γ=aS/ϕA |
| γ | interfacial tension; |
| γs | the interfacial tension in a blend having the interface fully saturated with the copolymer; |
| γ0 | interfacial tension between homopolymers without compatibilization; |
| ηm | viscosity of the matrix; |
| ηd | viscosity of the dispersed phase; |
| μ | chemical potential (usually with subscripts denoting component and superscripts denoting phase or system type); |
| π | the Ludolf number; |
| φA, φB, φCD | volume fractions of the A homopolymer, B homopolymer and the copolymer in the system; |
| φk | volume fraction in the k phase, where k is the A or B bulk phase or the interface (I); |
| volume fraction relative to φA, e.g., = φCD/φA; | |
| copolymer amount at the interface; | |
| relative copolymer amount at the interface; | |
| χAB | the Flory–Huggins interaction parameter between the homopolymer blocks A and B; |
| χij | the Flory–Huggins interaction parameter between the block j and the homopolymer i; |
| Ψ | the parameter related to the copolymer content at the interface, defined in Equation (5); |
| ∇s | the surface gradient operator; |
| cr | critical; |
| cyl | cylindrical; |
| DMS | dimethylsiloxane; |
| eq | equilibrium; |
| HDPE | high density polyethylene; |
| LDPE | low density polyethylene; |
| LDPE2 | low density polyethylene different from that denoted LDPE; |
| lam | lamellar; |
| Mic | relating to the micelle; |
| ODT | order–disorder transition; |
| PCHMA | poly(cyclohexyl methacrylate); |
| PDMS | polydimethylsiloxane; |
| PMMA | poly(methyl methacrylate); |
| PO | polyolefin; |
| PP | polypropylene; |
| PS | polystyrene; |
| PS-b-PMMA | poly(styrene-b-methyl methacrylate); |
| rpm | revolutions per minute; |
| SB | styrene-butadiene; |
| SCF | self-consistent field; |
| sph | spherical. |
References
- Hudson, S.D.; Jamieson, A.M. Morphology and properties of blends containing block copolymers. In Polymer Blends, Vol. 1: Formulations; Paul, D.R., Bucknall, C.B., Eds.; J. Wiley and Sons: New York, NY, USA, 2000; pp. 461–499. ISBN 978-0-471-24825-5. [Google Scholar]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Covas, J.A.; Pessan, L.A.; Machado, A.V.; Larocca, N.M. Ch. 7: Polymer blend compatibilization by copolymers and functional polymers. In Encyclopedia of Polymer Blends, Vol. 2: Processing; Isayev, A.I., Ed.; Wiley-VCH: Weinheim, Germany, 2011; Volume 2, pp. 315–356. [Google Scholar] [CrossRef]
- Anastasiadis, S.H. Interfacial tension in binary polymer blends and the effects of copolymers as emulsifying agents. In Polymer thermodynamics. Advances in Polymer Science, vol 238; Wolf, B., Enders, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 179–269. [Google Scholar] [CrossRef]
- Favis, B.D. Factor influencing the morphology in immiscible polymer blends in melt processing. In Polymer Blends; Paul, D.R., Bucknall, C.B., Eds.; J. Wiley and Sons: New York, NY, USA, 2000; Volume 1, pp. 501–537. [Google Scholar] [CrossRef]
- Fortelný, I. Theoretical aspects of phase morphology development. In Micro- and Nanostructured Multiphase Polymer Blends Systems; Harrats, C., Thomas, S., Groeninckx, G., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2006; pp. 43–90. [Google Scholar] [CrossRef]
- Sundararaj, U. Phase morphology development in polymer blends. In Micro- and Nanostructured Multiphase Polymer Blends Systems; Harrats, C., Thomas, S., Groeninckx, G., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2006; pp. 133–164. [Google Scholar] [CrossRef]
- Huang, H.-X. Macro, micro and nanostructured morphologies of multiphase polymer systems. In Handbook of Multiphase Polymer Systems; Boudenne, A., Ibos, L., Candau, Y., Thomas, S., Eds.; Wiley: Chichester, UK, 2011; Volume 1, pp. 161–249. [Google Scholar] [CrossRef]
- Fortelný, I.; Jůza, J. Description of the Droplet Size Evolution in Flowing Immiscible Polymer Blends. Polymers 2019, 11, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glansdorf, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability and Fluctuations; Wiley-Interscience: London, UK, 1971. [Google Scholar]
- Lyngaae-Jørgensen, J. Diblock copolymers and steric stabilization during flow. J. Macromol. Sci. Phys. 1998, B37, 239–253. [Google Scholar] [CrossRef]
- Tang, T.; Huang, B. Interfacial behaviour of compatibilizers in polymer blends. Polymer 1994, 35, 281–285. [Google Scholar] [CrossRef]
- Kim, J.R.; Jamieson, A.M.; Hudson, S.D.; Manas-Zloczower, I.; Ishida, H. Influence of Segmental Swelling Ratio of a Symmetric Block Copolymer on the Morphology of Melt-Mixed Immiscible Polymer Blends. Macromolecules 1999, 32, 4582–4587. [Google Scholar] [CrossRef]
- Chun, S.B.; Han, C.D. The Role of the Order−Disorder Transition Temperature of Block Copolymer in the Compatibilization of Two Immiscible Homopolymers. Macromolecules 1999, 32, 4030–4042. [Google Scholar] [CrossRef]
- Adedeji, A.; Lyu, S.; Macosko, C.W. Block Copolymers in Homopolymer Blends: Interface vs. Micelles. Macromolecules 2001, 34, 8663–8668. [Google Scholar] [CrossRef]
- Wang, J.; Tsou, A.H.; Passino, H.L.; Favis, B.D. PPE-g-HDPE in high-performance poly(p-phenylene ether)/polyethylene blends: Synthesis and compatibilization effects. Polymer 2018, 138, 92–102. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Rayón, E.; Samper, M.D.; Balart, R. Compatibilization and Characterization of Polylactide and Biopolyethylene Binary Blends by Non-Reactive and Reactive Compatibilization Approaches. Polymers 2020, 12, 1344. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Y.; Kong, M.; Yang, Q.; Li, G. Assessment of compatibilization efficiency of SEBS in the PP/PS blend. J. Appl. Polym. Sci. 2018, 135, 46244. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, W.; Huang, D.; Lu, B.; Wang, P.; Wang, G.; Ji, J. Compatibilization of immiscible PLA-based biodegradable polymer blends using amphiphilic di-block copolymers. Eur. Polym. J. 2019, 118, 45–52. [Google Scholar] [CrossRef]
- Leibler, L. Emulsifying effects of block copolymers in incompatible polymer blends. Makromol. Chemie. Macromol. Symp. 1988, 16, 1–17. [Google Scholar] [CrossRef]
- Retsos, H.; Margiolaki, I.; Messaritaki, A.A.; Anastasiadis, S.H. Interfacial Tension in Binary Polymer Blends in the Presence of Block Copolymers: Effects of Additive MW. Macromolecules 2001, 34, 5295–5305. [Google Scholar] [CrossRef]
- Noolandi, J. Interfacial tension in incompatible homopolymer blends with added block copolymer. Makromol. Chem. Rapid Commun. 1991, 12, 517–521. [Google Scholar] [CrossRef]
- Retsos, H.; Anastasiadis, S.H.; Pispas, S.; Mays, J.W.; Hadjichristidis, N. Interfacial Tension in Binary Polymer Blends in the Presence of Block Copolymers. 2. Effects of Additive Architecture and Composition. Macromolecules 2004, 37, 524–537. [Google Scholar] [CrossRef]
- Erukhimovich, I.; Govorun, E.N.; Litmanovich, A.D. Stabilization of polymer blend structure by diblock copolymers. Macromol. Theory Simul. 1998, 7, 233–239. [Google Scholar] [CrossRef]
- Govorun, E.N.; Erukhimovich, I. Emulsion Stabilization by Diblock Copolymers: Droplet Curvature Effect. Langmuir 1999, 15, 8392–8398. [Google Scholar] [CrossRef]
- Lyatskaya, Y.; Gersappe, D.; Gross, N.A.; Balazs, A.C. Designing Compatibilizers To Reduce Interfacial Tension in Polymer Blends. J. Phys. Chem. 1996, 100, 1449–1458. [Google Scholar] [CrossRef]
- Scheutjens, J.M.H.M.; Fleer, G.J. Statistical theory of the adsorption of interacting chain molecules. 1. Partition function, segment density distribution, and adsorption isotherms. J. Phys. Chem. 1979, 83, 1619–1635. [Google Scholar] [CrossRef]
- Lyatskaya, Y.; Jacobson, S.H.; Balazs, A.C. Effect of Composition on the Compatibilizing Activity of Comb Copolymers. Macromolecules 1996, 29, 1059–1061. [Google Scholar] [CrossRef]
- Lyatskaya, Y.; Balazs, A.C. Using Copolymer Mixtures To Compatibilize Immiscible Homopolymer Blends. Macromolecules 1996, 29, 7581–7587. [Google Scholar] [CrossRef]
- Shull, K.R.; Kramer, E.J. Mean-field theory of polymer interfaces in the presence of block copolymers. Macromolecules 1990, 23, 4769–4779. [Google Scholar] [CrossRef]
- Shull, K.R.; Kramer, E.J.; Hadziioannou, G.; Tang, W. Segregation of block copolymers to interfaces between immiscible homopolymers. Macromolecules 1990, 23, 4780–4787. [Google Scholar] [CrossRef]
- Reynolds, B.J.; Ruegg, M.L.; Mates, T.E.; Radke, C.J.; Balsara, N.P. Experimental and Theoretical Study of the Adsorption of a Diblock Copolymer to Interfaces between Two Homopolymers. Macromolecules 2005, 38, 3872–3882. [Google Scholar] [CrossRef]
- Gersappe, D.; Harm, P.K.; Irvine, D.; Balazs, A.C. Contrasting the compatibilizing activity of comb and linear copolymers. Macromolecules 1994, 27, 720–724. [Google Scholar] [CrossRef]
- Reynolds, B.J.; Ruegg, M.L.; Mates, T.E.; Radke, C.J.; Balsara, N.P. Diblock Copolymer Surfactant Transport across the Interface between Two Homopolymers. Langmuir 2006, 22, 9192–9200. [Google Scholar] [CrossRef]
- Reynolds, B.J.; Ruegg, M.L.; Balsara, N.P.; Radke, C.J. Relationship between Macroscopic and Microscopic Models of Surfactant Adsorption Dynamics at Fluid Interfaces. Langmuir 2006, 22, 9201–9207. [Google Scholar] [CrossRef]
- Chang, K.; Morse, D.C. Diblock Copolymer Surfactants in Immiscible Homopolymer Blends: Swollen Micelles and Interfacial Tension. Macromolecules 2006, 39, 7746–7756. [Google Scholar] [CrossRef]
- Vilgis, T.A.; Noolandi, J. Theory of homopolymer-block copolymer blends. The search for a universal compatibilizer. Macromolecules 1990, 23, 2941–2947. [Google Scholar] [CrossRef]
- Adedeji, A.; Hudson, S.D.; Jamieson, A.M. Effect of Exothermic Interfacial Mixing on Interfacial Activity of a Block Copolymer. Macromolecules 1996, 29, 2449–2456. [Google Scholar] [CrossRef]
- Fortelný, I.; Jůza, J. Analysis of the effect of block copolymers on interfacial tension in immiscible polymer blends. Polymer 2018, 150, 380–390. [Google Scholar] [CrossRef]
- Noolandi, J.; Hong, K.M. Effect of block copolymers at a demixed homopolymer interface. Macromolecules 1984, 17, 1531–1537. [Google Scholar] [CrossRef]
- Semenov, A.N. Theory of diblock-copolymer segregation to the interface and free surface of a homopolymer layer. Macromolecules 1992, 25, 4967–4977. [Google Scholar] [CrossRef]
- Jůza, J.; Fortelný, I. Analysis of the effect of interaction parameters of copolymer blocks on their efficiency in reduction of interfacial tension in immiscible polymer blends. Colloid Polym. Sci. 2021, 299, 1247–1269. [Google Scholar] [CrossRef]
- Jůza, J.; Fortelný, I. Removal of some approximations in calculation of the effect of a block copolymer on the interfacial tension in polymer blends. Colloid Polym. Sci. 2021, 1–20. [Google Scholar] [CrossRef]
- Noolandi, J. Multiblock copolymers as polymeric surfactants: Are “pancakes” better than “dumbbells”? Die Makromol. Chem. Theory Simul. 1992, 1, 295–298. [Google Scholar] [CrossRef]
- Dai, K.H.; Kramer, E.J.; Shull, K.R. Interfacial segregation in two-phase polymer blends with diblock copolymer additives: The effect of homopolymer molecular weight. Macromolecules 1992, 25, 220–225. [Google Scholar] [CrossRef]
- Bačová, P.; Foskinis, R.; Glynos, E.; Rissanou, A.N.; Anastasiadis, S.H.; Harmandaris, V. Effect of macromolecular architecture on the self-assembly behavior of copolymers in a selective polymer host. Soft Matter 2018, 14, 9562–9570. [Google Scholar] [CrossRef]
- Whitmore, M.D.; Noolandi, J. Theory of micelle formation in block copolymer-homopolymer blends. Macromolecules 1985, 18, 657–665. [Google Scholar] [CrossRef]
- Leibler, L.; Orland, H.; Wheeler, J.C. Theory of critical micelle concentration for solutions of block copolymers. J. Chem. Phys. 1983, 79, 3550–3557. [Google Scholar] [CrossRef]
- Fortelný, I. Breakup and Coalescence of Dispersed Droplets in Compatibilized Polymer Blends. J. Macromol. Sci. Phys. B 2000, B39, 67–78. [Google Scholar] [CrossRef]
- Van Puyvelde, P.; Velankar, S.; Moldenaers, P. Rheology and morphology of compatibilized polymer blends. Curr. Opin. Colloid Interface Sci. 2001, 6, 457–463. [Google Scholar] [CrossRef]
- Gabriele, M.; Pasquino, R.; Grizzuti, N. Effects of Viscosity-Controlled Interfacial Mobility on the Coalescence of Immiscible Polymer Blends. Macromol. Mater. Eng. 2011, 296, 263–269. [Google Scholar] [CrossRef]
- Flumerfelt, R.W. Effects of dynamic interfacial properties on drop deformation and orientation in shear and extensional flow fields. J. Colloid Interface Sci. 1980, 76, 330–349. [Google Scholar] [CrossRef]
- Stone, H.A. Dynamics of Drop Deformation and Breakup in Viscous Fluids. Annu. Rev. Fluid Mech. 1994, 26, 65–102. [Google Scholar] [CrossRef]
- Nagarajan, R. Constructing a molecular theory of self-assembly: Interplay of ideas from surfactants and block copolymers. Adv. Colloid Interface Sci. 2017, 244, 113–123. [Google Scholar] [CrossRef]
- Stone, H.A.; Leal, L.G. The effects of surfactants on drop deformation and breakup. J. Fluid Mech. 1990, 220, 161–186. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.; Han, C.C. Lateral Mobility of Single Chains at a Liquid Polymer Interface. Macromolecules 2008, 41, 7284–7286. [Google Scholar] [CrossRef]
- Cox, R.G. The deformation of a drop in a general time-dependent fluid flow. J. Fluid Mech. 1969, 37, 601–623. [Google Scholar] [CrossRef]
- Abbassi-Sourki, F.; Huneault, M.A.; Bousmina, M. Effect of compatibilization on the deformation and breakup of drops in step-wise increasing shear flow. Polymer 2009, 50, 645–653. [Google Scholar] [CrossRef]
- Velankar, S.; Van Puyvelde, P.; Mewis, J.; Moldenaers, P. Effect of compatibilization on the breakup of polymeric drops in shear flow. J. Rheol. 2001, 45, 1007–1019. [Google Scholar] [CrossRef] [Green Version]
- Van Puyvelde, P.; Velankar, S.; Mewis, J.; Moldenaers, P. Effect of marangoni stresses on the deformation and coalescence in compatibilized immiscible polymer blends. Polym. Eng. Sci. 2002, 42, 1956–1964. [Google Scholar] [CrossRef]
- Jeon, H.K.; Macosko, C.W. Visualization of block copolymer distribution on a sheared drop. Polymer 2003, 44, 5381–5386. [Google Scholar] [CrossRef]
- Cardinaels, R.; Vananroye, A.; Van Puyvelde, P.; Moldenaers, P. Breakup Criteria for Confined Droplets: Effects of Compatibilization and Component Viscoelasticity. Macromol. Mater. Eng. 2011, 296, 231–242. [Google Scholar] [CrossRef]
- Vananroye, A.; Van Puyvelde, P.; Moldenaers, P. Deformation and orientation of single droplets during shear flow: Combined effects of confinement and compatibilization. Rheol. Acta 2011, 50, 231–242. [Google Scholar] [CrossRef]
- Hu, Y.T.; Pine, D.J.; Leal, L.G. Drop deformation, breakup, and coalescence with compatibilizer. Phys. Fluids 2000, 12, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Palierne, J.F.; Lequeux, F. Sausage instability of a thread in a matrix; linear theory for viscoelastic fluids and interface. J. Non-Newtonian Fluid Mech. 1991, 40, 289–306. [Google Scholar] [CrossRef]
- Tomotika, S. On the instability of a cylindrical thread of a viscous liquid surrounded by another viscous fluid. Proc. R. Soc. 1935, 150, 322–337. [Google Scholar] [CrossRef]
- Chesters, A.K. The modeling of coalescence processes in fluid-liquid dispersions: A review of current understanding. Trans. Inst. Chem. Eng. (A) 1991, 69, 259–270. [Google Scholar]
- Janssen, P.J.A.; Anderson, P.D. Modeling Film Drainage and Coalescence of Drops in a Viscous Fluid. Macromol. Mater. Eng. 2011, 296, 238–248. [Google Scholar] [CrossRef]
- Milner, S.T.; Xi, H. How copolymers promote mixing of immiscible homopolymers. J. Rheol. 1996, 40, 663–687. [Google Scholar] [CrossRef]
- Jeelani, S.A.K.; Hartland, S. Effect of Interfacial Mobility on Thin Film Drainage. J. Colloid Interface Sci. 1994, 164, 296–308. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W. Drop Breakup and Coalescence in Polymer Blends: The Effects of Concentration and Compatibilization. Macromolecules 1995, 28, 2647–2657. [Google Scholar] [CrossRef]
- Macosko, C.W.; Guégan, P.; Khandpur, A.K.; Nakayama, A.; Marechal, P.; Inoue, T. Compatibilizers for Melt Blending: Premade Block Copolymers. Macromolecules 1996, 29, 5590–5598. [Google Scholar] [CrossRef]
- Lyu, S.; Jones, T.D.; Bates, F.S.; Macosko, C.W. Role of Block Copolymers on Suppression of Droplet Coalescence. Macromolecules 2002, 35, 7845–7855. [Google Scholar] [CrossRef]
- Lyu, S. Block Copolymers Suppressing Droplet Coalescence through Stopping Film Rupture. Macromolecules 2003, 36, 10052–10055. [Google Scholar] [CrossRef]
- Vannozzi, C. Relaxation and coalescence of two equal-sized viscous drops in a quiescent matrix. J. Fluid Mech. 2012, 694, 408–425. [Google Scholar] [CrossRef]
- Wang, H.; Zinchenko, A.Z.; Davis, R.H. The collision rate of small drops in linear flow fields. J. Fluid Mech. 1994, 265, 161–188. [Google Scholar] [CrossRef]
- Hudson, S.D.; Jamieson, A.M.; Burkhart, B.E. The effect of surfactant on the efficiency of shear-induced drop coalescence. J. Colloid Interface Sci. 2003, 265, 409–421. [Google Scholar] [CrossRef]
- Cristini, V.; Bławzdziewicz, J.; Loewenberg, M. Near-contact motion of surfactant-covered spherical drops. J. Fluid Mech. 1998, 366, 259–287. [Google Scholar] [CrossRef]
- Bławzdziewicz, J.; Cristini, V.; Loewenberg, M. Near-Contact Motion of Surfactant-Covered Spherical Drops: Ionic Surfactant. J. Colloid Interface Sci. 1999, 211, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Chesters, A.K.; Bazhlekov, I.B. Effect of Insoluble Surfactants on Drainage and Rupture of a Film between Drops Interacting under a Constant Force. J. Colloid Interface Sci. 2000, 230, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.W.; Yoon, Y.; Leal, L.G. The effect of compatibilizer on the coalescence of two drops in flow. Phys. Fluids 2003, 15, 849–867. [Google Scholar] [CrossRef]
- Fortelný, I. An analysis of the origin of coalescence suppression in compatibilized polymer blends. Eur. Polym. J. 2004, 40, 2161–2166. [Google Scholar] [CrossRef]
- Fortelný, I.; Jůza, J.; Dimzoski, B. Coalescence in quiescent polymer blends with a high content of the dispersed phase. Eur. Polym. J. 2012, 48, 1230–1240. [Google Scholar] [CrossRef]
- Fortelný, I.; Živný, A. Theoretical description of steady droplet size in polymer blends containing a compatibilizer. Polymer 2000, 41, 6865–6873. [Google Scholar] [CrossRef]
- Janssen, J.M.H.; Meijer, H.E.H. Dynamics of liquid-liquid mixing: A 2-zone model. Polym. Eng. Sci. 1995, 35, 1766–1780. [Google Scholar] [CrossRef] [Green Version]
- Fortelný, I.; Kovář, J. Droplet size of the minor component in the mixing of melts of immiscible polymers. Eur. Polym. J. 1989, 25, 317–319. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Giacchi, G.; Mistretta, M.C.; Botta, L. Effect of a Compatibilizer on the Morphology and Properties of Polypropylene/Polyethylentherephthalate Spun Fibers. Polymers 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Cigana, P.; Favis, B.D.; Jerome, R. Diblock copolymers as emulsifying agents in polymer blends: Influence of molecular weight, architecture, and chemical composition. J. Polym. Sci. Part B: Polym. Phys. 1996, 34, 1691–1700. [Google Scholar] [CrossRef]
- Favis, B.D.; Cigana, P.; Matos, M.; Trembla, A. Factors influencing the efficacy of an interfacial modifier for the interface in an immiscible polymer blend. Can. J. Chem. Eng. 1997, 75, 273–281. [Google Scholar] [CrossRef]
- Matos, M.; Favis, B.D.; Lomellini, P. Interfacial modification of polymer blends—the emulsification curve: 1. Influence of molecular weight and chemical composition of the interfacial modifier. Polymer 1995, 36, 3899–3907. [Google Scholar] [CrossRef]
- Li, J.; Favis, B.D. Strategies to measure and optimize the migration of the interfacial modifier to the interface in immiscible polymer blends. Polymer 2002, 43, 4935–4945. [Google Scholar] [CrossRef]
- Cerclé, C.; Favis, B.D. Generalizing interfacial modification in polymer blends. Polymer 2012, 53, 4338–4343. [Google Scholar] [CrossRef]
- Marić, M.; Macosko, C.W. Block copolymer compatibilizers for polystyrene/poly(dimethylsiloxane) blends. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 346–357. [Google Scholar] [CrossRef]
- Fortelný, I.; Hlavatá, D.; Mikešová, J.; Michálková, D.; Potroková, L.; Šloufová, I. Effect of mixing conditions on the morphology and properties of polystyrene/polyethylene blends compatibilized with styrene-butadiene block copolymers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 609–622. [Google Scholar] [CrossRef]
- Fortelný, I.; Mikešová, J.; Hromádková, J.; Hašová, V.; Horák, Z. Effect of molecular structure of styrene-butadiene block copolymers on morphology, rheological properties, and impact strength of polystyrene/polyethylene blends. J. Appl. Polym. Sci. 2003, 90, 2303–2309. [Google Scholar] [CrossRef]
- Fortelný, I.; Šlouf, M.; Hlavatá, D.; Sikora, A. Interfacial activity of styrene-butadiene block copolymers in low-density polyethylene/polystyrene blends. Compos. Interfaces 2006, 13, 783–799. [Google Scholar] [CrossRef]
- Fortelný, I.; Šlouf, M.; Sikora, A.; Hlavatá, D.; Hašová, V.; Mikešová, J.; Jacob, C. The effect of the architecture and concentration of styrene–butadiene compatibilizers on the morphology of polystyrene/low-density polyethylene blends. J. Appl. Polym. Sci. 2006, 100, 2803–2816. [Google Scholar] [CrossRef]
- Fortelný, I.; Minkova, L.I.; Kotek, J.; Lapčíková, M.; Michálková, D. Morphology and mechanical properties of polypropylene/polystyrene blends compatibilized with styrene-butadiene block copolymers. Polym. Eng. Sci. 2012, 52, 191–204. [Google Scholar] [CrossRef]
- Hlavatá, D.; Hromádková, J.; Fortelný, I.; Hašová, V.; Pulda, J. Compatibilization efficiency of styrene-butadiene triblock copolymers in polystyrene-polypropylene blends with varying compositions. J. Appl. Polym. Sci. 2004, 92, 2431–2441. [Google Scholar] [CrossRef]
- Rek, V.; Vranješ, N.; Šlouf, M.; Fortelný, I.; Jelčić, Ž. Morphology and Properties of SEBS Block Copolymer Compatibilized PS/HDPE Blends. J. Elastomers Plast. 2008, 40, 237–251. [Google Scholar] [CrossRef]
- Starý, Z.; Fortelný, I.; Kruliš, Z.; Šlouf, M. Effect of the molecular structure of ethene–propene and styrene–butadiene copolymers on their compatibilization efficiency in low-density polyethylene/polystyrene blends. J. Appl. Polym. Sci. 2008, 107, 174–186. [Google Scholar] [CrossRef]
- Horák, Z.; Hlavatá, D.; Fortelný, I.; Lednický, F. Effect of styrene-butadiene triblock copolymer structure on its compatibilization efficiency in PS/PB and PS/PP blends. Polym. Eng. Sci. 2002, 42, 2042–2047. [Google Scholar] [CrossRef]
- Hlavatá, D.; Horák, Z.; Lednický, F.; Hromádková, J.; Pleska, A.; Zanevskii, Y.V. Compatibilization efficiency of styrene-butadiene multiblock copolymers in PS/PP blends. J. Polym. Sci. Part B: Polym. Phys. 2001, 39, 931–942. [Google Scholar] [CrossRef]









| Model | Density of a Copolymerat the Interface | Stretching of Copolymer Blocks | Ni/Pi Ratio for Applicability of the Model |
|---|---|---|---|
| Wet mushroom | Very low | Unstretched | Whole range |
| Wet brush | Low | Stretched | High, Ni > Pi3/2 |
| Dry brush | High | Stretched | Small, Ni < Pi3/2 |
| Feature | Distance of Initiation | More Important for | Dependence on the Rate of Droplets Approach | Note |
|---|---|---|---|---|
| Marangoni effect | Droplet radius | Low copolymer density at the interface | Increase with the rate | |
| Steric repulsion | Copolymer end-to-end distance | High copolymer density at the interface | Independent of the rate | Negative correlation with the Marangoni effect |
| PS Matrix—PMMA Particles | Affecting Particle Size | ||
|---|---|---|---|
| p = ηd/ηm | Ni vs. Pi | During Mixing | After Annealing |
| 10 to 30 | Ni ≈ Pi | No effect | Almost stable |
| 10 to 30 | Ni >> Pi | Increases | Does not stabilize |
| 0.3 to 2 | Reduces | Does not stabilize | |
| PS Matrix—PDMS Particles | Affecting Particle Size | ||
| p | Nivs.Pi | During Mixing | After Annealing |
| 0.03 to 0.05 | Ni < Pi | Reduces | Does not stabilize |
| 0.03 to 0.05 | Ni > Pi | no | Does not stabilize |
| 0.3 | Ni ≈ Pi | no | Stabilizes |
| 2.6 | NS > PPS, NDMS << PPDMS | Reduces | Stabilizes |
| 2.6 | Other than above | Reduces | Does not stabilize |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortelný, I.; Jůza, J. The Effects of Copolymer Compatibilizers on the Phase Structure Evolution in Polymer Blends—A Review. Materials 2021, 14, 7786. https://doi.org/10.3390/ma14247786
Fortelný I, Jůza J. The Effects of Copolymer Compatibilizers on the Phase Structure Evolution in Polymer Blends—A Review. Materials. 2021; 14(24):7786. https://doi.org/10.3390/ma14247786
Chicago/Turabian StyleFortelný, Ivan, and Josef Jůza. 2021. "The Effects of Copolymer Compatibilizers on the Phase Structure Evolution in Polymer Blends—A Review" Materials 14, no. 24: 7786. https://doi.org/10.3390/ma14247786