Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of Biohybrids Based on Turmeric-Generated Ag/AgClNPs
2.2.1. Preparation of Biomimetic Membranes
2.2.2. Preparation of Silver/Silver Chloride Nanoparticles
2.2.3. Eco-Design of Biohybrids
2.3. Physico-Chemical and Biological Characterization of Developed Materials
2.3.1. Spectral, Structural, and Morphological Characterization
2.3.2. In Vitro Antioxidant Activity
2.3.3. Antibacterial Assay of Tested Samples
2.3.4. Cell Viability
2.3.5. Morphological Evaluation of Cells
2.3.6. Hemocompatibility
2.4. Statistical Analysis
3. Results and Discussion
3.1. Optical Characterization of “Green” Developed Materials
3.2. Structural and Morphological Characterization of the Silver-Based Biohybrids
3.2.1. Small-Angle Neutron and X-ray Scattering
3.2.2. X-ray Diffraction
3.2.3. AFM Analysis of the Silver-Based Materials
3.2.4. SEM/EDS Investigations of Silver-Containing Materials
3.3. Evaluation of Zeta Potential of the Silver-Based Particles
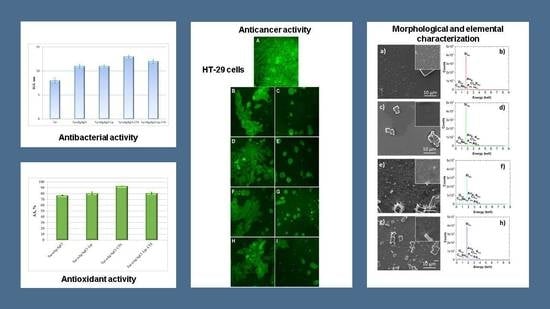
3.4. Evaluation of Antioxidant and Antibacterial Activities of Bio-Designed Materials
3.5. Evaluation of Antitumoral Properties of Turmeric-Generated Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gahlawat, G.A.; Choudhury, R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [Green Version]
- Espenti, C.S.; Rao, K.S.V.K.; Rao, K.M. Biosynthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential. Mater. Lett. 2016, 174, 129–133. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, Q.; Sun, D.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; Hong, J.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Kumar, V.V.; Yadov, S.C.; Yadov, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces 2017, 158, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.W. Nanomaterials Formed by Green Nanotechnology for Bioapplication; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 241–310. [Google Scholar]
- Kailasa, S.K.; Park, T.J.; Rohit, J.V.; Koduru, J.R. Antimicrobial activity of silver nanoparticles. In Nanoparticles in Pharmacotherapy; William Andrew Publishing: Philadelphia, PA, USA, 2019; pp. 461–484. [Google Scholar]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Nazir, A. Green synthesis of metal nanoparticles and their applications in different fields: A review. Z. Phys. Chem. 2019, 233, 1325–1349. [Google Scholar] [CrossRef]
- Mubarak Ali, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, M.S.; Zvezdanović, J.B.; Stanojević, L.P.; Stanojević, J.S.; Petrović, S.M.; Cakić, M.D.; Cvetković, D.J. Synthesis, characterization and antioxidant activity of silver nanoparticles stabilized by aqueous extracts of wild blackberry (Rubus spp.) and raspberry (Rubus idaeus L.) leaves. Adv. Technol. 2019, 8, 47–58. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Mallikarjuna, K.; Sushma, N.J.; Narasimha, G.; Manoj, L.; Raju, D.P. Phytochemical fabrication and characterization of silver nanoparticles by using pepper leaf broth. Arab. J. Chem. 2014, 7, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Sharma, S.; Singh, V.N.; Shamsi, S.F.; Fatma, A.; Mehta, B.R. Biosynthesis of Silver Nanoparticles from Desmodium triflorum: A Novel Approach Towardsweed Utilization; SAGE-Hindawi Access to Research Biotechnology Research International: Thousand Oaks, CA, USA, 2011; pp. 2011–2018. [Google Scholar]
- Ullah, N.; Li, D.; Xiaodong, C.; Yasin, S.; Muhammed Umair, M.; Shan Van Eede, S.; Wei, Q. Photo-irradiation based biosynthesis of silver nanoparticles by using an ever green shrub and its antibacterial study. Dig. J. Nanomater. Biostruct. 2015, 10, 95–105. [Google Scholar]
- Liu, Y.; Hussain, M.; Memon, H.; Yasin, S. Solar irradiation and Nageia nagi extract assisted rapid synthesis of silver nanoparticles and their antibacterial activity. Dig. J. Nanomater. Biostruct. 2015, 10, 1019–1024. [Google Scholar]
- Yu, L.; Memon, H.; Bhavsar, P.; Yasin, S. Fabrication of alginate fibers loaded with silver nanoparticles biosynthesized via dolcetto grape leaves (Vitis vinifera cv.): Morphological, antimicrobial characterization and in vitro release studies. Mater. Focus 2016, 5, 216–221. [Google Scholar] [CrossRef]
- Maghimaa, M.; Alharbi, S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabric for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B 2020, 204, 111806. [Google Scholar] [CrossRef]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Allafchian, A.R.; Jalali, S.A.H.; Aghaei, F.; Farhang, H.R. Green synthesis of silver nanoparticles using Glaucium corniculatum (L.) Curtis extract and evaluation of its antibacterial activity. IET Nanobiotechnol. 2018, 12, 574–578. [Google Scholar] [CrossRef]
- Sheikh, E.; Bhatt, M.B.; Tripathi, M. Bio-based synthesised and characterized monodispersed Curcuma longa silver nanoparticles induces targeted anticancer activity in breast cancer cells. Pharmacogn. Mag. 2018, 14, 340. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.D.; Nhuan, N.T.; Van, L.T.; Minh, N.V.; Anh, N.P.; Tri, N. Biofabrication of silver nanoparticles using Curcuma longa extract: Effects of extraction and synthesis conditions, characteristics, and its antibacterial activity. J. Biochem. Technol. 2020, 11, 57–66. [Google Scholar]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Selvan, D.A.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B 2018, 180, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Rahman, P.K.; Varunkumar, K.; Anusha, C.; Kalaiarasi, A.; Shivashangari, K.S.; Ravikumar, V. Facile synthesis of Curcuma longa tuber powder engineered metal nanoparticles for bioimaging applications. J. Mol. Struct. 2017, 1129, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Peng, H.; Qiu, L.; Wang, R.; Jiang, C.; Cai, T.; Sun, Y.; Li, Y.; Xiong, H. New pectin-induced green fabrication of Ag@AgCl/ZnO nanocomposites for visible-light triggered antibacterial activity. Int. J. Biol. Macromol. 2019, 141, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical potential of ultrafine Ag/AgCl nanoparticles coated on graphene with special reference to antimicrobial performances and burn wound healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef]
- Kota, S.; Dumpala, P.; Anantha, R.K.; Verma, M.K.; Kandepu, S. Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Sci. Rep. 2017, 7, 11566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Ferreira, V.; Ferreira Conz Eugenio, M.; Del Nery dos Santos, E.; de Souza, W.; Sant’Anna, C. Cellular toxicology and mechanism of the response to silver-based nanoparticle exposure in Ewing’s sarcoma cells. Nanotechnology 2021, 32, 11510. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf. B Biointerfaces 2018, 171, 398–405. [Google Scholar] [CrossRef]
- Khan, M.J.; Shameli, K.; Sazili, A.Q.; Selamat, J.; Kumari, S. Rapid Green Synthesis and Characterization of Silver Nanoparticles Arbitrated by Curcumin in an Alkaline Medium. Molecules 2019, 24, 719. [Google Scholar] [CrossRef] [Green Version]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Ogasawara, A.A.; Iwasa, T.; Blom, J.; Sekimizu, K. Complete genome sequence and comparative genomic analysis of Enterococcus faecalis EF-2001, a probiotic bacterium. Genomics 2021, 113, 1534–1542. [Google Scholar] [CrossRef]
- Bhonchal Bhardwaj, S. Enterococci: An Important Nosocomial Pathogen, Pathogenic Bacteria, Sahra Kırmusaoğlu and Sonia Bhonchal Bhardwaj; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Strain, H.H.; Svec, W.A.; Vernon, L.P.; Seely, G.R. The Chlorophylls; Academic Press: New York, NY, USA, 1966; Volume 2, pp. 21–66. [Google Scholar]
- Gorshkova, Y.; Barbinta-Patrascu, M.-E.; Bokuchava, G.; Badea, N.; Ungureanu, C.; Lazea-Stoyanova, A.; Răileanu, M.; Bacalum, M.; Turchenko, V.; Zhigunov, A.; et al. Biological Performances of Plasmonic Biohybrids Based on Phyto-Silver/Silver Chloride Nanoparticles. Nanomaterials 2021, 11, 1811. [Google Scholar] [CrossRef]
- Nyam-Osor, M.; Soloviov, D.V.; Kovalev, Y.S.; Zhigunov, A.; Rogachev, A.V.; Ivankov, O.I.; Erhan, R.V.; Kuklin, A.I. Silver behenate and silver stearate powders for calibration of SAS instruments. J. Phys. Conf. Ser. 2012, 351, 012024. [Google Scholar] [CrossRef]
- Kieffer, J.; Karkoulis, D. PyFAI, a versatile library for azimuthal regrouping. J. Phys. Conf. Ser. 2013, 425, 202012. [Google Scholar] [CrossRef]
- Hammouda, B. A new Guinier-Porod model. J. Appl. Cryst. 2010, 43, 716–771. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Islamov, A.K.; Gordeliy, V.I. Scientific Reviews: Two-Detector System for Small-Angle Neutron Scattering Instrument. Neutron News 2005, 16, 16. [Google Scholar] [CrossRef]
- Soloviev, A.G.; Murugova, T.N.; Islamov, A.K.; Kuklin, A.I. FITTER. The package for fitting a chosen theoretical multi-parameter function through a set of data points. Application to experimental data of the YuMO spectrometer. J. Phys. Conf. Ser. 2012, 351, 012027. [Google Scholar] [CrossRef]
- Ostanevich, Y.M. Makromol. Chem. Macromol. Symp. 1988, 15, 91. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Badea, N.; Ţugulea, L.; Giurginca, M.; Meghea, A. Oxidative stress simulation on artificial membranes- chemiluminescent studies. Rev. Chim. 2008, 59, 834–837. [Google Scholar]
- Ungureanu, C.; Ferdes, M. Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv. Sci. Lett. 2012, 18, 50–53. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed.; CLSI Document M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, Approved Guideline; CLSI Document M44-A; CLSI: Wayne, PA, USA, 2004. [Google Scholar]
- Barbinta-Patrascu, M.E.; Badea, N.; Bacalum, M.; Ungureanu, C.; Suica-Bunghez, I.R.; Iordache, S.M.; Pirvu, C.; Zgura, I.; Maraloiu, V.A. 3D hybrid structures based on biomimetic membranes and Caryophyllus aromaticus—“Green” synthesized nano-silver with improved bioperformances. Mat. Sci. Eng. C 2019, 101, 120–137. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Besliu, D.; Lazea-Stoyanova, A.; Iosif, L. Bio-active nanomaterials phyto-generated from weed herb Cirsium arvense. Optoelectron. Adv. Mater. 2020, 14, 459–465. [Google Scholar]
- Barbinta-Patrascu, M.E.; Badea, N.; Ungureanu, C.; Ispas, A. Photophysical aspects regarding the effects of Paeonia officinalis flower extract on DNA molecule labelled with methylene blue. Optoelectron. Adv. Mater. Rapid Commun. 2019, 13, 131–135. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; JohnWiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Balachandran, Y.L.; Girija, S.; Selvakumar, R.; Tongpim, S.; Gutleb, A.C.; Suriyanarayanan, S. differently environment stable bio-silver nanoparticles: Study on their optical enhancing and antibacterial properties. PLoS ONE 2013, 8, e77043. [Google Scholar]
- Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Santana, R.V.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of mesoporous silica nanoparticles on glycerol-plasticized anionic and cationic polysaccharide edible films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Phanjom, P.; Ahmed, G. Biosynthesis of silver nanoparticles by Aspergillus oryzae (MTCC no. 1846) and its characterizations. Nanosci. Nanotechnol. 2015, 5, 14–21. [Google Scholar]
- He, Y.; Du, Z.; Lv, H.; Jia, Q.; Tang, Z.; Zheng, X.; Zhang, K.; Zhao, F. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat extract and their application in clinical ultrasound gel. Int. J. Nanomed. 2013, 8, 1809–1815. [Google Scholar] [CrossRef] [Green Version]
- Barbinta-Patrascu, M.E.; Badea, N.; Pirvu, C.; Bacalum, M.; Ungureanu, C.; Nadejde, P.L.; Ion, C.; Rau, I. Multifunctional soft hybrid bio-platforms based on nano-silver and natural compounds. Mater. Sci. Eng. C 2016, 69, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Badea, N.; Bacalum, M.; Lazea-Stoyanova, A.; Zgura, I.; Negrila, C.; Enculescu, M.; Burnei, C. Novel Ecogenic Plasmonic Biohybrids as Multifunctional Bioactive Coatings. Coatings 2020, 10, 659. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Iordache, S.M.; Iordache, A.M.; Bunghez, I.R.; Ghiurea, M.; Badea, N.; Fierascu, R.C.; Stamatin, I. Eco-designed biohybrids based on liposomes, mint—Nanosilver and carbon nanotubes for antioxidant and antimicrobial coating. Mater. Sci. Eng. C 2014, 39, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Colton, R.H.; Henn, D.E. Crystal structure of disodium orthophosphite pentahydrate. J. Chem. Soc. A 1971, 1207–1209. [Google Scholar] [CrossRef]
- Schmidt, P.W.; Avnir, D.; Levy, D.; Hohr, A.; Steiner, M.; Roll, A. Small Angle X-Ray Scattering from the Surfaces of Reversed Phase Silicas: Power-Law Scattering Exponents of Magnitudes Greater than Four. J. Chem. Phys. 1991, 94, 1474. [Google Scholar] [CrossRef]
- Ryan, K.J.; Ray, C.G. Sherris Medical Microbiology, 4th ed.; McGraw Hill: New York, NY, USA, 2004; pp. 294–295. ISBN 0-8385-8529-9. [Google Scholar]
- Huycke, M.M.; Spiegel, C.A.; Gilmore, M.S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 1991, 35, 1626–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocas, I.; Siqueira, J.; Santos, K. Association of Enterococcus faecalis With Different Forms of Periradicular Diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef]
- Lyu, Y.; Yu, M.; Liu, Q.; Zhang, Q.; Liu, Z.; Tian, Y.; Li, D.; Changdao, M. Synthesis of silver nanoparticles using oxidized amylose and combination with curcumin for enhanced antibacterial activity. Carbohydr. Polym. 2020, 230, 115573. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Subbarao, C.; Sharma, S.; Subbarao, C.V.; Garcia-Godoy, F.; Gutmann, J.L. Effectiveness of curcumin against Enterococcus faecalis biofilm. Acta Odontol Scand. 2013, 71, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, R.; Bhat, S.S.; Sundeep Hegde, K. Antibacterial Activity of Turmeric against Enterococcus faecalis—An in vitro Study. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 498–504. [Google Scholar]














| Biohybrid Complex | Rg2, nm | S2 | m2 | Rg1, nm | s1 | m1 | DSAXS,2, nm | DSAXS,1, nm | DXRD_AgCl, nm | DXRD_Ag, nm |
|---|---|---|---|---|---|---|---|---|---|---|
| Tur-nAg/AgCl | 44.7 | 0.2 | 4.8 | 10.8 | 1.2 | 3.6 | 115.3 | 27.8 | 102 | 24 |
| Tur-nAg/AgCl–Lip | 48.1 | 0.2 | 4.7 | 12.9 | 0.8 | 3.6 | 124.1 | 33.3 | 113 | 34 |
| Tur-nAg/AgCl–CTS | 44.8 | 0 | 4.4 | 11.6 | 1.3 | 3.9 | 115.6 | 29.9 | 97 | 26 |
| Tur-nAg/AgCl–Lip–CTS | 44.6 | 0 | 4.2 | 11.1 | 1.4 | 3.9 | 115.1 | 28.6 | 95 | 25 |
| Samples | IC50/mg/mL | TI | |||
|---|---|---|---|---|---|
| BJ | HT-29 | HepG2 | HT-29 | HepG2 | |
| Tur-nAg/AgCl | 36.41 | 41.53 | 44.6 | 0.88 | 0.82 |
| Tur-nAg/AgCl–Lip | 36.93 | 20.97 | 36.52 | 1.76 | 1.01 |
| Tur-nAg/AgCl–CTS | 39.28 | 39.69 | 46.03 | 0.99 | 0.85 |
| Tur-nAg/AgCl–Lip–CTS | 33.04 | 28.03 | 27.72 | 1.18 | 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbinta-Patrascu, M.-E.; Gorshkova, Y.; Ungureanu, C.; Badea, N.; Bokuchava, G.; Lazea-Stoyanova, A.; Bacalum, M.; Zhigunov, A.; Petrovic, S. Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles. Materials 2021, 14, 4726. https://doi.org/10.3390/ma14164726
Barbinta-Patrascu M-E, Gorshkova Y, Ungureanu C, Badea N, Bokuchava G, Lazea-Stoyanova A, Bacalum M, Zhigunov A, Petrovic S. Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles. Materials. 2021; 14(16):4726. https://doi.org/10.3390/ma14164726
Chicago/Turabian StyleBarbinta-Patrascu, Marcela-Elisabeta, Yulia Gorshkova, Camelia Ungureanu, Nicoleta Badea, Gizo Bokuchava, Andrada Lazea-Stoyanova, Mihaela Bacalum, Alexander Zhigunov, and Sanja Petrovic. 2021. "Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles" Materials 14, no. 16: 4726. https://doi.org/10.3390/ma14164726