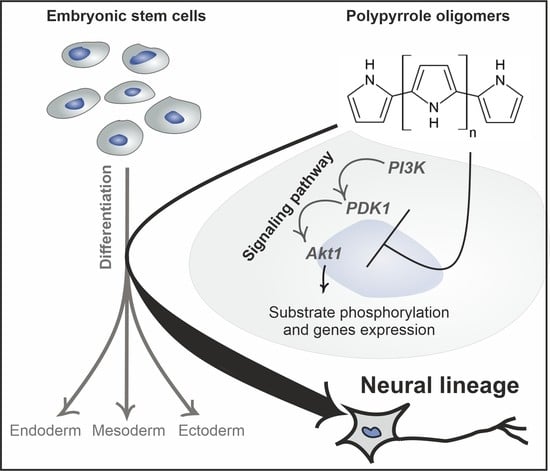
Modulation of Differentiation of Embryonic Stem Cells by Polypyrrole: The Impact on Neurogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PPy Powders
2.2. Preparation of Extracts
2.3. Culture of Embryonic Stem Cells
2.4. Cytotoxicity
2.5. ESCs Differentiation
2.6. Expressions of Neural Markers by Quantitative RT-PCR
2.7. Western Blot
2.8. Characterization of Extracts
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPs | conducting polymers |
| ESCs | embryonic stem cells |
| PPy | polypyrrole |
| NT3 | neurotrophin-3 |
| LIF | leukemia inhibitory factor |
| EBs | embryoid bodies |
| ITS | Insulin–transferrin–selen |
| REF | reference |
| PBAT | butylene adipate-co-terephthalate |
| hNSCs | human neural stem cells |
| PLCL/SF | composite of poly (L-lactic acid-co-ɛ-caprolactone) with silk fibroin |
| CNS | central nervous system |
| MAPs | microtubule-associated proteins |
| GSK-3 | glycogen synthase kinase-3 |
| MS | mass spectrometry |
References
- Ateh, D.; Navsaria, H.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, K.-M.; Hoffman-Kim, D.; Song, H.-K.; Palmore, G.T.R. Quantitative Control of Neuron Adhesion at a Neural Interface Using a Conducting Polymer Composite with Low Electrical Impedance. ACS Appl. Mater. Interfaces 2011, 3, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; Schmidt, C.E. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for en-hanced neurite extension. J. Biomed. Mater. Res. Part A 2007, 81, 135–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittnacht, U.; Hartmann, H.; Hein, S.; Oliveira, H.; Dong, M.; Pêgo, A.P.; Kjems, J.; Howard, K.A.; Schlosshauer, B. Chitosan/siRNA Nanoparticles Biofunctionalize Nerve Implants and Enable Neurite Outgrowth. Nano Lett. 2010, 10, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tanemura, K.; Okada, S.; Iwanami, A.; Nakamura, M.; Mizuno, H.; Ozawa, M.; Ohyama-Goto, R.; Kitamura, N.; Kawano, M.; et al. Electrical Stimulation Modulates Fate Determination of Differentiating Embryonic Stem Cells. Stem Cells 2006, 25, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonner, J.M.; Forciniti, L.; Nguyen, H.; Byrne, J.D.; Kou, Y.F.; Syeda-Nawaz, J.; Schmidt, C.E. Biocompatibility implications of polypyrrole synthesis techniques. Biomed. Mater. 2008, 3, 034124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Castano, H.; O’Rear, E.A.; McFetridge, P.S.; Sikavitsas, V.I. Polypyrrole Thin Films Formed by Admicellar Polymeriza-tion Support the Osteogenic Differentiation of Mesenchymal Stem Cells. Macromol. Biosci. 2004, 4, 785–794. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole salts and bases: Superior conductivity of nanotubes and their stability towards the loss of conductivity by depro-tonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Serna, F.; Nickels, A.J.; Schmidt, C.E. Carboxylic Acid-Functionalized Conductive Polypyrrole as a Bioactive Platform for Cell Adhesion. Biomacromolecules 2006, 7, 1692–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, K.J.; Kita, M.; Han, Y.; Gelmi, A.; Higgins, M.J.; Moulton, S.E.; Clark, G.M.; Kapsa, R.; Wallace, G.G. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials 2009, 30, 5292–5304. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Thompson, B.; Moulton, S.E.; Newbold, C.; Lum, M.G.; Cameron, A.; Wallace, G.G.; Kapsa, R.; Clark, G.; O’Leary, S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials 2007, 28, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Bio-compatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, E.J.; Park, W.K.; Kim, H.J.; Jeong, D.; Jung, M.E.; Song, K.S.; Lee, S.H.; Seo, H.J.; Kim, M.J.; et al. Arylpiperazine-containing pyrrole 3-carboxamide derivatives targeting serotonin 5-HT2A, 5-HT2C, and the serotonin transporter as a potential antidepressant. Bioorganic Med. Chem. Lett. 2010, 20, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Bavadi, M.; Niknam, K.; Shahraki, O. Novel pyrrole derivatives bearing sulfonamide groups: Synthesis in vitro cytotoxicity evaluation, molecular docking and DFT study. J. Mol. Struct. 2017, 1146, 242–253. [Google Scholar] [CrossRef]
- Aiello, A.; D’Esposito, M.; Fattorusso, E.; Menna, M.; Müller, W.E.G.; Perović-Ottstadt, S.; Tsuruta, H.; Gulder, T.A.M.; Bringmann, G. Daminin, a bioactive pyrrole alkaloid from the Mediterranean sponge Axinella damicornis. Tetrahedron 2005, 61, 7266–7270. [Google Scholar] [CrossRef]
- Ahmad, S.; Alam, O.; Naim, M.J.; Shaquiquzzaman, M.; Alam, M.M.; Iqbal, M. Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur. J. Med. Chem. 2018, 157, 527–561. [Google Scholar] [CrossRef]
- Humpolíček, P.; Kašpárková, V.; Pacherník, J.; Stejskal, J.; Bober, P.; Capáková, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocký, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, em-bryotoxicity and impurity profile. Mater. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef]
- Nagy, A.; Rossant, J.; Abramow-Newerly, W.; Roder, J.C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 1993, 90, 8424–8428. [Google Scholar] [CrossRef] [Green Version]
- Humpolíček, P.; Radaszkiewicz, K.A.; Kašpárková, V.; Stejskal, J.; Trchová, M.; Kuceková, Z.; Vičarová, H.; Pacherník, J.; Lehocký, M.; Minařík, A. Stem cell differentiation on conducting polyaniline. RSC Adv. 2015, 5, 68796–68805. [Google Scholar] [CrossRef] [Green Version]
- Bober, P.; Humpolíček, P.; Pacherník, J.; Stejskal, J.; Lindfors, T. Conducting polyaniline based cell culture substrate for embryonic stem cells and embryoid bodies. RSC Adv. 2015, 5, 50328–50335. [Google Scholar] [CrossRef] [Green Version]
- Konopka, R.; Hýzdalová, M.; Kubala, L.; Pacherník, J. New luminescence-based approach to measurement of luciferase gene expression reporter activity and adenosine triphosphate-based determination of cell viability. Folia Biol. 2010, 56, 66–71. [Google Scholar]
- Willems, E.; Mateizel, I.; Kemp, C.; Cauffman, G.; Sermon, K.; Leyns, L. Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int. J. Dev. Biol. 2006, 50, 627–635. [Google Scholar] [CrossRef]
- Abranches, E.; Silva, M.; Pradier, L.; Schulz, H.; Hummel, O.; Henrique, D.; Bekman, E. Neural Differentiation of Embryonic Stem Cells In Vitro: A Road Map to Neurogenesis in the Embryo. PLoS ONE 2009, 4, e6286. [Google Scholar] [CrossRef]
- Večeřa, J.; Kudová, J.; Kučera, J.; Kubala, L.; Pacherník, J. Neural Differentiation Is Inhibited through HIF1α/β-Catenin Signaling in Embryoid Bodies. Stem Cells Int. 2017, 2017, 8715798. [Google Scholar] [CrossRef]
- Kučera, J.; Binó, L.; Štefková, K.; Jaroš, J.; Vašíček, O.; Večeřa, J.; Kubala, L.; Pacherník, J. Apocynin and Diphe-nyleneiodonium Induce Oxidative Stress and Modulate PI3K/Akt and MAPK/Erk Activity in Mouse Embryonic Stem Cells. Oxidative Med. Cell. Longev. 2016, 2016, 7409196. [Google Scholar] [CrossRef] [Green Version]
- Binó, L.; Veselá, I.; Papežíková, I.; Procházková, J.; Vašíček, O.; Štefková, K.; Kučera, J.; Hanáčková, M.; Ku-bala, L.; Pacherník, J. The depletion of p38alpha kinase upregulates NADPH oxidase 2/NOX2/gp91 expression and the pro-duction of superoxide in mouse embryonic stem cells. Arch. Biochem. Biophys. 2019, 671, 18–26. [Google Scholar] [CrossRef]
- Chen, J.; Zacharek, A.; Li, Y.; Li, A.; Wang, L.; Katakowski, M.; Roberts, C.; Lu, M.; Chopp, M. N-cadherin me-diates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience 2006, 140, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Fiszbein, A.; Schor, I.E.; Kornblihtt, A.R. Fundamentals of NCAM Expression, Function, and Regulation of Alternative Splicing in Neuronal Differentiation. In Neural Surface Antigens; Pruszak, J., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 131–140. [Google Scholar]
- Fanarraga, M.L.; Avila, J.; Zabala, J.C. Expression of unphosphorylated class III beta-tubulin isotype in neuroepithelial cells demonstrates neuroblast commitment and differentiation. Eur. J. Neurosci. 1999, 11, 516–527. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Park, P.J. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Fourniol, F.; Perderiset, M.; Houdusse, A.; Moores, C. Chapter 3—Structural Studies of the Doublecortin Family of MAPs. In Methods in Cell Biology; Correia, J.J., Wilson, L., Eds.; Academic Press: Boston, MA, USA, 2013; Volume 115, pp. 27–48. [Google Scholar]
- She, X.; Rohl, C.A.; Castle, J.C.; Kulkarni, A.; Johnson, J.M.; Chen, R. Definition, conservation and epigenetics of housekeeping and tissue-enriched genes. BMC Genom. 2009, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capáková, Z.; Radaszkiewicz, K.A.; Acharya, U.; Truong, T.H.; Pacherník, J.; Bober, P.; Kašpárková, V.; Stejskal, J.; Pfleger, J.; Lehocký, M.; et al. The biocompatibility of polyaniline and polypyrrole 211.Doping with organic phosphonates. Mater. Sci. Eng. C 2020, 113, 110986. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Nichols, J.; Robertson, M.; Rathjen, P.D. Differentiation inhibiting activity (DIA/LIF) and mouse development. Dev. Biol. 1992, 151, 339–351. [Google Scholar] [CrossRef]
- Cerdan, C.; Hong, S.H.; Bhatia, M. Formation and Hematopoietic Differentiation of Human Embryoid Bodies by Suspen-sion and Hanging Drop Cultures. Curr. Protoc. Stem Cell Biol. 2007, 3, 1D.2.1–1D.2.16. [Google Scholar] [CrossRef]
- Granato, A.E.C.; Ribeiro, A.C.; Marciano, F.R.; Rodrigues, B.V.M.; Lobo, A.O.; Porcionatto, M. Polypyrrole in-creases branching and neurite extension by Neuro2A cells on PBAT ultrathin fibers. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1753–1763. [Google Scholar] [CrossRef]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.C.; Moulton, S.E.; Kapsa, R.; Wallace, G.G.; Crook, J.M. Electrical Stimulation Using Conductive Polymer Polypyrrole Promotes Differentiation of Human Neural Stem Cells: A Biocompatible Platform for Translational Neural Tissue Engineering. Tissue Eng. Part C Methods 2015, 21, 385–393. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompat-ibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 411–422. [Google Scholar] [CrossRef]
- Sun, B.; Wu, T.; Wang, J.; Li, D.; Wang, J.; Gao, Q.; Bhutto, M.A.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous membranes promoting neural cell proliferation and differentiation with electrical stimulation. J. Mater. Chem. B 2016, 4, 6670–6679. [Google Scholar] [CrossRef]
- Simpson, T.I.; Price, D.J. Pax6: A pleiotropic player in development. BioEssays 2002, 24, 1041–1051. [Google Scholar] [CrossRef]
- Pevny, L.H.; Sockanathan, S.; Placzek, M.; Lovell-Badge, R. A role for SOX1 in neural determination. Development 1998, 125, 1967–1978. [Google Scholar] [PubMed]
- Lo, L.; Tiveron, M.C.; Anderson, D.J. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development 1998, 125, 609–620. [Google Scholar] [PubMed]
- Kumar, N.; Afeyan, R.; Sheppard, S.; Harms, B.; Lauffenburger, D.A. Quantitative analysis of Akt phosphorylation and activity in response to EGF and insulin treatment. Biochem. Biophys. Res. Commun. 2007, 354, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kučera, J.; Netušilová, J.; Sladeček, S.; Lánová, M.; Vašíček, O.; Štefková, K.; Navrátilová, J.; Kubala, L.; Pacherník, J. Hypoxia Downregulates MAPK/ERK but Not STAT3 Signaling in ROS-Dependent and HIF-1-Independent Manners in Mouse Embryonic Stem Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E. Inhibition of mitogen-activated protein kinase and stimulation of Akt kinase signaling pathways: Two approaches with therapeutic potential in the treatment of neurodegenerative disease. Pharmacol. Ther. 2007, 114, 261–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, D.A.E.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nat. Cell Biol. 1995, 378, 785–789. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [Green Version]
- Eldar-Finkelman, H.; Martinez, A. GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS. Front. Mol. Neurosci. 2011, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Pap, M.; Cooper, G.M. Role of Glycogen Synthase Kinase-3 in the Phosphatidylinositol 3-Kinase/Akt Cell Survival Pathway. J. Biol. Chem. 1998, 273, 19929–19932. [Google Scholar] [CrossRef] [Green Version]
- Coghlan, M.P.; Culbert, A.A.; Cross, D.A.E.; Corcoran, S.L.; Yates, J.W.; Pearce, N.J.; Rausch, O.L.; Mur-phy, G.J.; Carter, P.S.; Roxbee Cox, L.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metab-olism and gene transcription. Chem. Biol. 2000, 7, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Kramer, T.; Schmidt, B.; Monte, F.L. Small-Molecule Inhibitors of GSK-3: Structural Insights and Their Application to Alzheimer’s Disease Models. Int. J. Alzheimer’s Dis. 2012, 2012, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.C.; Ng, S.; Ni, Z.J.; Pfister, K.B.; Ramurthy, S.; Subramanian, S.; Wagman, A.S. Pyrrole Based Inhibitors of Glycogen Synthase Kinase 3. U.S. Patent No. 7,250,443, 31 July 2007. [Google Scholar]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seira, O.; del Río, J.A. Glycogen Synthase Kinase 3 Beta (GSK3β) at the Tip of Neuronal Development and Regeneration. Mol. Neurobiol. 2014, 49, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Kotasová, H.; Veselá, I.; Kučera, J.; Houdek, Z.; Procházková, J.; Králičková, M.; Pacherník, J. Phosphoinositide 3-kinase inhibition enables retinoic acid-induced neurogenesis in monolayer culture of embryonic stem cells. J. Cell. Biochem. 2012, 113, 563–570. [Google Scholar] [CrossRef]
- Wataya, T.; Ando, S.; Muguruma, K.; Ikeda, H.; Watanabe, K.; Eiraku, M.; Kawada, M.; Takahashi, J.; Hashimoto, N.; Sasai, Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 11796–11801. [Google Scholar] [CrossRef] [Green Version]
- Pacherník, J.; Esner, M.; Bryja, V.; Dvořak, P.; Hampl, A. Neural differentiation of mouse embryonic stem cells grown in monolayer. Reprod. Nutr. Dev. 2003, 42, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Stejskal, J.; Hajná, M.; Kašpárková, V.; Humpolíček, P.; Zhigunov, A.; Trchová, M. Purification of a conducting polymer, polyaniline, for biomedical applications. Synth. Met. 2014, 195, 286–293. [Google Scholar] [CrossRef]
- Kašpárková, V.; Humpolíček, P.; Stejskal, J.; Kopecká, J.; Kuceková, Z.; Moučka, R. Conductivity, impurity profile, and cytotoxicity of solvent-extracted polyaniline. Polym. Adv. Technol. 2016, 27, 156–161. [Google Scholar] [CrossRef]
- Appel, G.; Schmeißer, D.; Bauer, J.; Bauer, M.; Egelhaaf, H.J.; Oelkrug, D. The formation of oligomers in the electrolyte upon polymerization of pyrrole. Synth. Met. 1999, 99, 69–77. [Google Scholar] [CrossRef]
- Fermin, D.; Scharifker, B. Products in solution during electrodeposition of polypyrrole. J. Electroanal. Chem. 1993, 357, 273–287. [Google Scholar] [CrossRef]
- Ozeki, Y.; Omae, M.; Kitagawa, S.; Ohtani, H. Electrospray ionization-ion mobility spectrometry-high resolution tandem mass spectrometry with collision-induced charge stripping for the analysis of highly multiply charged intact polymers. Analyst 2019, 144, 3428–3435. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, N.H.; Kim, N.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.-T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wei, X.; Xu, L.; Li, Z.; Liu, G.; Zhang, X. A New Natural Lactone from Dimocarpus longan Lour. Seeds. Molecules 2012, 17, 9421–9425. [Google Scholar] [CrossRef]
- Reddy, C.R.; Tukaram, A.G.; Mohammed, S.Z.; Dilipkumar, U.; Babu, B.N.; Chakravarty, S.; Bhattacharya, D.; Joshi, P.; Gree, R. Synthesis and biological evaluation of longanlactone analogues as neurotrophic agents. Bioorganic Med. Chem. Lett. 2018, 28, 673–676. [Google Scholar] [CrossRef] [PubMed]








| Gene | Primer Sequence | T annealing (°C) | Product Size (pb) |
|---|---|---|---|
| Pax 6 | F TGCCCTTCCATCTTTGCTTG | 60 | 178 |
| R TCTGCCCGTTCAACATCCTTAG | |||
| Sox 1 | F CCAGCCTCCAGAGCCCGACT | 62 | 258 |
| R GGCATCGCCTCGCTGGGTTT | |||
| Mash 1 | F GGTCTCGTCCTACTCCTCCG | 62 | 137 |
| R GCTGCCATCCTGCTTCCAAA | |||
| Gapdh | F AAGGGCTCATGACCACAGTC | 62 | 252 |
| R CATACTTGGCAGGTTTCTCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopalová, K.; Radaszkiewicz, K.A.; Kašpárková, V.; Stejskal, J.; Bober, P.; Junkar, I.; Mozetič, M.; Capáková, Z.; Lehocký, M.; Kašparová, M.; et al. Modulation of Differentiation of Embryonic Stem Cells by Polypyrrole: The Impact on Neurogenesis. Int. J. Mol. Sci. 2021, 22, 501. https://doi.org/10.3390/ijms22020501
Skopalová K, Radaszkiewicz KA, Kašpárková V, Stejskal J, Bober P, Junkar I, Mozetič M, Capáková Z, Lehocký M, Kašparová M, et al. Modulation of Differentiation of Embryonic Stem Cells by Polypyrrole: The Impact on Neurogenesis. International Journal of Molecular Sciences. 2021; 22(2):501. https://doi.org/10.3390/ijms22020501
Chicago/Turabian StyleSkopalová, Kateřina, Katarzyna Anna Radaszkiewicz, Věra Kašpárková, Jaroslav Stejskal, Patrycja Bober, Ita Junkar, Miran Mozetič, Zdenka Capáková, Marián Lehocký, Martina Kašparová, and et al. 2021. "Modulation of Differentiation of Embryonic Stem Cells by Polypyrrole: The Impact on Neurogenesis" International Journal of Molecular Sciences 22, no. 2: 501. https://doi.org/10.3390/ijms22020501