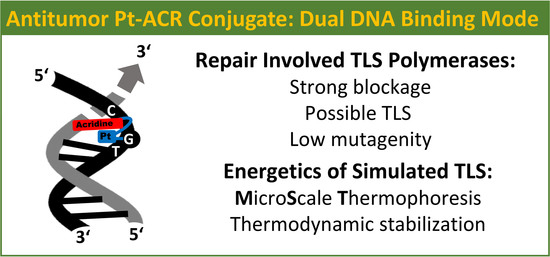
Processing and Bypass of a Site-Specific DNA Adduct of the Cytotoxic Platinum–Acridinylthiourea Conjugate by Polymerases Involved in DNA Repair: Biochemical and Thermodynamic Aspects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transcription Mapping of DNA–ACR Adducts
2.2. Enzymatic Translesion Synthesis Assays
2.2.1. Running-Start Primer Extension Experiments
2.2.2. Standing-Start Primer Extension Experiments
2.2.3. Nucleotide Misinsertion Opposite the ACR Adduct by KFexo− and Human Polymerases Eta, Kappa, or Iota
2.3. Simulated TLS by Microscale Thermophoresis (MST)
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Transcription Mapping of DNA Platinum Adducts
4.3. Platination of Oligonucleotides
4.4. Translesion Synthesis Assays
4.5. Nucleotide Misinsertion by KFexo− and Human Polymerases Eta, Kappa, Iota
4.6. Steady-State Kinetic Analysis for dNTP Incorporations by KFexo− and Polη
4.7. MST-Derived Thermodynamic Parameters
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography |
| GF AA | Graphite furnace atomic absorption spectrometry |
| EAS | Electronic absorption spectrophotometry |
| TLS | Translesion DNA synthesis |
| KFexo− | Klenow fragment of DNA polymerase I (the exonuclease deficient) |
| PAA | Polyacrylamide |
| MST | Microscale thermophoresis |
| DTT | Dithiothreitol |
| BSA | Bovine serum albumin |
| dNTP | 2′-deoxyribonucleotide-5′-triphosphate |
| dNMP | 2′-deoxyribonucleotide-5′-monophosphate |
| ACR | [PtCl(en)(L)](NO3)2 (en = ethane-1,2-diamine, L = 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea) complex |
| AMD | [PtCl(en)(L)](NO3)2 (en = ethane-1,2-diamine, L = N-[2-(acridin-9-ylamino)ethyl]-N-methylpropionamidine) complex |
| cisplatin | cis-[Pt(NH3)2Cl2] (cis-diamminedichloridoplatinum(II)) |
| Cy5 | Cyanine dye (1,1′-bis(3-hydroxypro-pyl)-3,3,3′,3′-tetramethylindodicarbocyanine) |
| pol | Polymerase |
| nt | Nucleotide |
| bp | Base pair |
References
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt (II) Agents, Nanoparticle Delivery, and Pt (IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.C.; Lippard, S.J. Inhibition of transcription by platinum antitumor compounds. Metallomics 2009, 1, 280–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brabec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic platinum coordination compounds. DNA binding agents. Coord. Chem. Rev. 2017, 351, 2–31. [Google Scholar] [CrossRef]
- Baruah, H.; Barry, C.G.; Bierbach, U. Platinum-intercalator conjugates: From DNA-targeted cisplatin derivatives to adenine binding complexes as potential modulators of gene regulation. Curr. Top. Med. Chem. 2004, 4, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Pierre, V.C.; Barton, J.K. Metallo-intercalators and metallo-insertors. Chem. Commun. 2007, 44, 4565–4579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, C.G.; Baruah, A.H.; Bierbach, U. Unprecedented Monofunctional Metalation of Adenine Nucleobase in Guanine- and Thymine-Containing Dinucleotide Sequences by a Cytotoxic Platinum−Acridine Hybrid Agent. J. Am. Chem. Soc. 2003, 125, 9629–9637. [Google Scholar] [CrossRef] [PubMed]
- Guddneppanavar, R.; Bierbach, U. Adenine-N3 in the DNA minor groove—An emerging target for platinum containing anticancer pharmacophores. Anti-Cancer Agents Med. Chem. 2007, 7, 125–138. [Google Scholar] [CrossRef]
- Budiman, M.E.; Alexander, R.W.; Bierbach, U. Unique base-step recognition by a platinum-acridinylthiourea conjugate leads to a DNA damage profile complementary to that of the anticancer drug cisplatin. Biochemistry 2004, 43, 8560–8567. [Google Scholar] [CrossRef]
- Baruah, H.; Wright, M.W.; Bierbach, U. Solution structural study of a DNA duplex containing the guanine-N7 adduct formed by a cytotoxic platinum-acridine hybrid agent. Biochemistry 2005, 44, 6059–6070. [Google Scholar] [CrossRef]
- Bassett, E.; Vaisman, A.; Havener, J.M.; Masutani, C.; Hanaoka, F.; Chaney, S.G. Efficiency of extension of mismatched primer termini across from cisplatin and oxaliplatin adducts by human DNA polymerases beta and eta in vitro. Biochemistry 2003, 42, 14197–14206. [Google Scholar] [CrossRef]
- Arana, M.E.; Song, L.; Le Gac, N.T.; Parris, D.S.; Villani, G.; Boehmer, P.E.B. On the role of proofreading exonuclease in bypass of a 1,2 d(GpG) cisplatin adduct by the herpes simplex virus-1 DNA polymerase. DNA Repair 2004, 3, 659–669. [Google Scholar] [CrossRef]
- Wickramaratne, S.; Boldry, E.J.; Buehler, C.; Wang, Y.-C.; Distefano, M.D.; Tretyakova, N.Y. Error-prone translesion synthesis past DNA-peptide cross-links conjugated to the major groove of DNA via C5 of thymidine. J. Biol. Chem. 2015, 290, 775–787. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, D.K.; Guengerich, F.P.; Egli, M.; Wilds, C.J. Backbone flexibility influences nucleotide incorporation by human translesion DNA polymerase η opposite intrastrand cross-linked DNA. Biochemistry 2015, 54, 7449–7456. [Google Scholar] [CrossRef] [Green Version]
- Villani, G.; Hubscher, U.; Gironis, N.; Parkkinen, S.; Pospiech, H.; Shevelev, I.; di Cicco, G.; Markkanen, E.; Syvaoja, J.E.; Tanguy Le Gac, N. In vitro gap-directed translesion DNA synthesis of an abasic site involving human DNA polymerases epsilon, lambda, and beta. J. Biol. Chem. 2011, 286, 32094–32104. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.V.; Guainazzi, A.; Derkunt, S.B.; Enoiu, M.; Schärer, O.D. Structure-dependent bypass of DNA interstrand crosslinks by translesion synthesis polymerases. Nucleic Acids Res. 2011, 39, 7455–7464. [Google Scholar] [CrossRef] [Green Version]
- Beard, W.A.; Wilson, S.H. Structural insights into the origins of DNA polymerase fidelity. Structure 2003, 11, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Kasparkova, J.; Suchankova, T.; Halamikova, A.; Zerzankova, L.; Vrana, O.; Margiotta, N.; Natile, G.; Brabec, V. Cytotoxicity, cellular uptake, glutathione and DNA interactions of an antitumor large-ring PtII chelate complex incorporating the cis-1,4-diaminocyclohexane carrier ligand. Biochem. Pharmacol. 2010, 79, 552–564. [Google Scholar] [CrossRef] [Green Version]
- Novakova, O.; Farrell, N.P.; Brabec, V. Translesion DNA synthesis across double-base lesions derived from cross-links of an antitumor trinuclear platinum compound: Primer extension, conformational and thermodynamic studies. Metallomics 2018, 10, 132–144. [Google Scholar] [CrossRef]
- Hubscher, U.; Maga, G.; Spadari, S. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 2002, 71, 133–163. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Aggarwal, A.K.; Rechkoblit, O. Eukaryotic DNA polymerases. Curr. Opin. Struct. Biol. 2018, 53, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Weiss, S.J.; Fisher, P.A. Recognition and binding of template-primers containing defined abasic sites by Drosophila DNA polymerase alpha holoenzyme. J. Biol. Chem. 1989, 264, 13018–13023. [Google Scholar] [CrossRef]
- Weiss, S.J.; Fisher, P.A. Interaction of Drosophila DNA polymerase alpha holoenzyme with synthetic template-primers containing mismatched primer bases or propanodeoxyguanosine adducts at various positions in template and primer regions. J. Biol. Chem. 1992, 267, 18520–18526. [Google Scholar] [CrossRef]
- Lindsley, J.E.; Fuchs, R.P.P. Use of single-turnover kinetics to study bulky adduct bypass by T7 DNA polymerase. Biochemistry 1994, 33, 764–772. [Google Scholar] [CrossRef]
- Miller, H.; Grollman, A.P. Kinetics of DNA polymerase I (Klenow fragment exo-) activity on damaged DNA templates: Effect of proximal and distal template damage on DNA synthesis. Biochemistry 1997, 36, 15336–15342. [Google Scholar] [CrossRef]
- Minetti, C.; Remeta, D.P.; Miller, H.; Gelfand, C.A.; Plum, G.E.; Grollman, A.P.; Breslauer, K.J. The thermodynamics of template-directed DNA synthesis: Base insertion and extension enthalpies. Proc. Natl. Acad. Sci. USA 2003, 100, 14719–14724. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Cho, B.P. Probing the thermodynamics of aminofluorene-induced translesion DNA synthesis by differential scanning calorimetry. J. Am. Chem. Soc. 2007, 129, 12108–12109. [Google Scholar] [CrossRef]
- Florian, J.; Brabec, V. Thermodynamics of translesion synthesis across a major DNA adduct of antitumor oxaliplatin: Differential scanning calorimetric study. Chem. Eur. J. 2012, 18, 1634–1639. [Google Scholar] [CrossRef]
- Turner, R.M.; Grindley, N.D.F.; Joyce, C.M. Interaction of DNA polymerase I (Klenow fragment) with the single-stranded template beyond the site of synthesis. Biochemistry 2003, 42, 2373–2385. [Google Scholar] [CrossRef]
- Patel, P.H.; Suzuki, M.; Adman, E.; Shinkai, A.; Loeb, L.A. Prokaryotic DNA polymerase I: Evolution, structure, and “base flipping” mechanism for nucleotide selection. J. Mol. Biol. 2001, 308, 823–837. [Google Scholar] [CrossRef] [Green Version]
- Villani, G.; Le Gac, N.T.; Wasungu, L.; Burnouf, D.; Fuchs, R.P.; Boehmer, P.E. Effect of manganese on in vitro replication of damaged DNA catalyzed by the herpes simplex virus type-1 DNA polymerase. Nucleic Acids. Res. 2002, 30, 3323–3332. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, A.; Masutani, C.; Hanaoka, F.; Chaney, S.G. Efficient translesion replication past oxaliplatin and cisplatin GpG adducts by human DNA polymerase η. Biochemistry 2000, 39, 4575–4580. [Google Scholar] [CrossRef]
- Bassett, E.; Vaisman, A.; Tropea, K.A.; McCall, C.M.; Masutani, C.; Hanaoka, F.; Chaney, S.G. Frameshifts and deletions during in vitro translesion synthesis past Pt-DNA adducts by DNA polymerases beta and eta. DNA Repair 2002, 1, 1003–1016. [Google Scholar] [CrossRef]
- Yamada, K.; Takezawa, J.; Ezaki, O. Translesion replication in cisplatin-treated xeroderma pigmentosum variant cells is also caffeine-sensitive: Features of the error-prone DNA polymerase(s) involved in UV-mutagenesis. DNA Repair 2003, 2, 909–924. [Google Scholar] [CrossRef]
- Bassett, E.; King, N.M.; Bryant, M.F.; Hector, S.; Pendyala, L.; Chaney, S.G.; Cordeiro-Stone, M. The role of DNA polymerase eta in translesion synthesis past platinum-DNA adducts in human fibroblasts. Cancer Res. 2004, 64, 6469–6475. [Google Scholar] [CrossRef] [Green Version]
- Albertella, M.R.; Green, C.M.; Lehmann, A.R.; O’Connor, M.J. A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 2005, 65, 9799–9806. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, A.; Woodgate, R. Translesion DNA polymerases in eukaryotes: What makes them tick? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 274–303. [Google Scholar] [CrossRef] [Green Version]
- Jha, V.; Ling, H. Structural basis for human DNA polymerase kappa to bypass cisplatin intrastrand cross-link (Pt-GG) lesion as an efficient and accurate extender. J. Mol. Biol. 2018, 430, 1577–1589. [Google Scholar] [CrossRef]
- Tissier, A.; McDonald, J.P.; Frank, E.G.; Woodgate, R. Polι, a remarkably error-prone human DNA polymerase. Genes Dev. 2000, 14, 1642–1650. [Google Scholar]
- McDonald, J.P.; Tissier, A.; Frank, E.G.; Iwai, S.; Hanaoka, F.; Woodgate, R. DNA polymerase iota and related Rad30-like enzymes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 53–60. [Google Scholar] [CrossRef]
- McIntyre, J. Polymerase iota-an odd sibling among Y family polymerases. DNA Repair 2020, 86, 102753. [Google Scholar] [CrossRef]
- Brown, J.A.; Newmister, S.A.; Fiala, K.A.; Suo, Z. Mechanism of double-base lesion bypass catalyzed by a Y-family DNA polymerase. Nucleic Acids Res. 2008, 36, 3867–3878. [Google Scholar] [CrossRef] [Green Version]
- Kostrhunova, H.; Malina, J.; Pickard, A.J.; Stepankova, J.; Vojtiskova, M.; Kasparkova, J.; Muchova, T.; Rohlfing, M.L.; Bierbach, U.; Brabec, V. Replacement of a thiourea with an amidine group in a monofunctional platinum-acridine antitumor agent. Effect on DNA interactions, DNA adduct recognition and repair. Mol. Pharm. 2011, 8, 1941–1954. [Google Scholar] [CrossRef] [Green Version]
- Pilch, D.S.; Dunham, S.U.; Jamieson, E.R.; Lippard, S.J.; Breslauer, K.J. DNA sequence context modulates the impact of a cisplatin 1,2-d(GpG) intrastrand cross-link and the conformational and thermodynamic properties of duplex DNA. J. Mol. Biol. 2000, 296, 803–812. [Google Scholar] [CrossRef]
- Hofr, C.; Farrell, N.; Brabec, V. Thermodynamic properties of duplex DNA containing a site-specific d(GpG) intrastrand cross-link formed by an antitumor dinuclear platinum complex. Nucleic Acids Res. 2001, 29, 2034–2040. [Google Scholar] [CrossRef] [Green Version]
- Malina, J.; Novakova, O.; Vojtiskova, M.; Natile, G.; Brabec, V. Conformation of DNA GG intrastrand cross-link of antitumor oxaliplatin and its enantiomeric analog. Biophys. J. 2007, 93, 3950–3962. [Google Scholar] [CrossRef] [Green Version]
- Bursova, V.; Kasparkova, J.; Hofr, C.; Brabec, V. Effects of monofunctional adducts of platinum (II) complexes on thermodynamic stability and energetics of DNA duplexes. Biophys. J. 2005, 88, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Malina, J.; Novakova, O.; Natile, G.; Brabec, V. The thermodynamics of translesion DNA synthesis past major adducts of enantiomeric analogues of antitumor cisplatin. Chem. Asian J. 2012, 7, 1026–1031. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; André, T.; Wanner, R.; Roth, H.M.; Duhr, S.; Baaske, P.; Breitsprecher, D. MicroScale Thermophoresis: Interaction analysis and beyond. J. Mol. Struct. 2014, 1077, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Hrabina, O.; Brabec, V.; Novakova, O. Translesion DNA synthesis across lesions induced by oxidative products of pyrimidines. An insight into the mechanism by microscale thermophoresis. Int. J. Mol. Sci. 2019, 20, 5012. [Google Scholar] [CrossRef] [Green Version]
- Hreusova, M.; Novakova, O.; Brabec, V. Thermodynamic insights by microscale thermophoresis into translesion DNA synthesis catalyzed by DNA polymerases across a lesion of antitumor platinum–acridine complex. Int. J. Mol. Sci. 2020, 21, 7806. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, M.A.; Schwartz, A.; Rahmouni, A.R.; Leng, M. Interstrand cross-links are preferentially formed at the d(GC) sites in the reaction between cis-diamminedichloroplatinum (II) and DNA. Proc. Natl. Acad. Sci. USA 1991, 88, 1982–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brabec, V.; Leng, M. DNA interstrand cross-links of trans-diamminedichloroplatinum (II) are preferentially formed between guanine and complementary cytosine residues. Proc. Natl. Acad. Sci. USA 1993, 90, 5345–5349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novakova, O.; Malina, J.; Kasparkova, J.; Halamikova, A.; Bernard, V.; Intini, F.; Natile, G.; Brabec, V. Energetics, conformation, and recognition of DNA duplexes modified by methylated analogues of [PtCl(dien)]+. Chem. Eur. J. 2009, 15, 6211–6221. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Salassa, L.; Habtemariam, A.; Novakova, O.; Pizarro, A.M.; Clarkson, G.J.; Liskova, B.; Brabec, V.; Sadler, P.J. Photoactivatable organometallic pyridyl ruthenium (II) arene complexes. Organometallics 2012, 31, 3466–3479. [Google Scholar] [CrossRef]
- Baruah, H.; Bierbach, U. Unusual intercalation of acridin-9-ylthiourea into the 5′-GA/TC DNA base step from the minor groove: Implications for the covalent DNA adduct profile of a novel platinum-intercalator conjugate. Nucleic Acids. Res. 2003, 31, 4138–4146. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Choudhury, J.R.; Wright, M.W.; Day, C.S.; Saluta, G.; Kucera, G.L.; Bierbach, U. A non-cross-linking platinum-acridine agent with potent activity in non-small-cell lung cancer. J. Med. Chem. 2008, 51, 7574–7580. [Google Scholar] [CrossRef] [Green Version]
- Kasparkova, J.; Novakova, O.; Marini, V.; Najajreh, Y.; Gibson, D.; Perez, J.-M.; Brabec, V. Activation of trans geometry in bifunctional mononuclear platinum complexes by a piperidine ligand: Mechanistic studies on antitumor action. J. Biol. Chem. 2003, 278, 47516–47525. [Google Scholar] [CrossRef] [Green Version]
- Comess, K.M.; Burstyn, J.N.; Essigmann, J.M.; Lippard, S.J. Replication inhibition and translesion synthesis on templates containing site-specifically placed cis-diamminedichloroplatinum (II) DNA adducts. Biochemistry 1992, 31, 3975–3990. [Google Scholar] [CrossRef]
- Suo, Z.; Johnson, K. DNA secondary structure effects on DNA synthesis catalyzed by HIV-1 reverse transcriptase. J. Biol. Chem. 1998, 273, 27259–27267. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, A.; Warren, M.W.; Chaney, S.G. The effect of DNA structure on the catalytic efficiency and fidelity of human DNA polymerase beta on templates with platinum-DNA adducts. J. Biol. Chem. 2001, 276, 18999–19005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriarity, B.; Novakova, O.; Farrell, N.; Brabec, V.; Kasparkova, J. 1,2-GG intrastrand cross-link of antitumor dinuclear bifunctional platinum compound with spermidine linker inhibits DNA polymerization more effectively than the cross-link of conventional cisplatin. Arch. Biochem. Biophys. 2007, 459, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Alt, A.; Lammens, K.; Chiocchini, C.; Lammens, A.; Pieck, J.C.; Kuch, D.; Hopfner, K.P.; Carell, T. Bypass of DNA lesions generated during anticancer treatment with cisplatin by DNA polymerase. Science 2007, 318, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Johnson, R.E.; Prakash, L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu. Rev. Biochem. 2005, 74, 317–353. [Google Scholar] [CrossRef]
- Creighton, S.; Bloom, L.B.; Goodman, M.F. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 1995, 262, 232–256. [Google Scholar]
- Mendelman, L.; Petruska, J.; Goodman, M. Base mispair extension kinetics. Comparison of DNA polymerase and reverse transcriptase. J. Biol. Chem. 1990, 265, 2338–2346. [Google Scholar] [CrossRef]
- Goodman, M.F.; Creighton, S.; Bloom, L.B.; Petruska, J.; Kunkel, T.A. Biochemical basis of DNA replication fidelity. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 83–126. [Google Scholar] [CrossRef]
- Baruah, H.; Bierbach, U. Biophysical characterization and molecular modeling of the coordinative-intercalative DNA monoadduct of a platinum-acridinylthiourea agent in a site-specifically modified dodecamer. J. Biol. Inorg. Chem. 2004, 9, 335–344. [Google Scholar] [CrossRef]
- Baruah, H.; Day, C.S.; Wright, M.W.; Bierbach, U. Metal-intercalator-mediated self-association and one-dimensional aggregation in the structure of the excised major DNA adduct of a platinum-acridine agent. J. Am. Chem. Soc. 2004, 126, 4492–4493. [Google Scholar] [CrossRef]
- Gill, M.R.; Thomas, J.A. Ruthenium (II) polypyridyl complexes and DNA-from structural probes to cellular imaging and therapeutics. Chem. Soc. Rev. 2012, 41, 3179–3192. [Google Scholar] [CrossRef]
- Bommarito, S.; Peyret, N.; SantaLucia, J. Thermodynamic parameters for DNA sequences with dangling ends. Nucleic Acids. Res. 2000, 28, 1929–1934. [Google Scholar] [CrossRef] [Green Version]
- Yakovchuk, P.; Protozanova, E.; Frank-Kamenetskii, M.D. Base-stacking and base-pairing contributions into thermal stability of the DNA double helix. Nucleic Acids. Res. 2006, 34, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Petruska, J.; Goodman, M.F.; Boosalis, M.S.; Sowers, L.C.; Cheong, C.; Tinoco, I., Jr. Comparison between DNA melting thermodynamics and DNA polymerase fidelity. Proc. Natl. Acad. Sci. USA 1988, 85, 6252–6256. [Google Scholar] [CrossRef] [Green Version]
- Kasparkova, J.; Mellish, K.J.; Qu, Y.; Brabec, V.; Farrell, N. Site-specific d(GpG) intrastrand cross-links formed by dinuclear platinum complexes. Bending and NMR studies. Biochemistry 1996, 35, 16705–16713. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hreusova, M.; Brabec, V.; Novakova, O. Processing and Bypass of a Site-Specific DNA Adduct of the Cytotoxic Platinum–Acridinylthiourea Conjugate by Polymerases Involved in DNA Repair: Biochemical and Thermodynamic Aspects. Int. J. Mol. Sci. 2021, 22, 10838. https://doi.org/10.3390/ijms221910838
Hreusova M, Brabec V, Novakova O. Processing and Bypass of a Site-Specific DNA Adduct of the Cytotoxic Platinum–Acridinylthiourea Conjugate by Polymerases Involved in DNA Repair: Biochemical and Thermodynamic Aspects. International Journal of Molecular Sciences. 2021; 22(19):10838. https://doi.org/10.3390/ijms221910838
Chicago/Turabian StyleHreusova, Monika, Viktor Brabec, and Olga Novakova. 2021. "Processing and Bypass of a Site-Specific DNA Adduct of the Cytotoxic Platinum–Acridinylthiourea Conjugate by Polymerases Involved in DNA Repair: Biochemical and Thermodynamic Aspects" International Journal of Molecular Sciences 22, no. 19: 10838. https://doi.org/10.3390/ijms221910838