Critical Analysis of Non-Thermal Plasma-Driven Modulation of Immune Cells from Clinical Perspective
Abstract
:1. Introduction
2. A Brief Physicochemical Characterization of NTP for Biomedicine
3. Critical Clinical View on NTP—Potential Side Effects and Clinical Validation
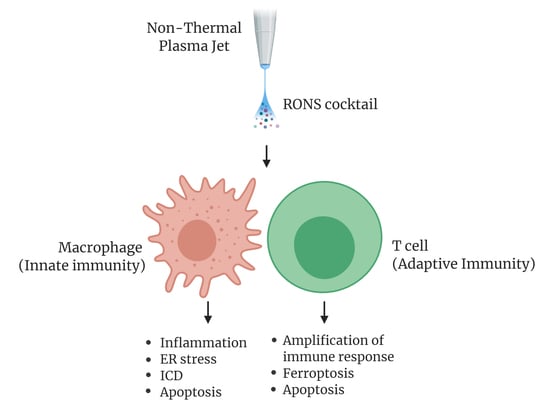
4. Effects of NTP on Immune Cells
5. Challenges in Deciphering Molecular Targets of NTP Action
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NTP | Non-thermal plasma |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| DBD | Dielectric barrier discharge |
| APPJ | Atmospheric pressure plasma jet |
| ROI | Reactive oxygen intermediates |
| CPGs | Clinical Practice Guidelines |
| ICD | Immunogenic cell death |
| ATP | Adenosine triphosphate |
| ATF4 | Activating transcription factor 4 |
| CAT | Catalase |
| CCL4 | Carbon tetrachloride |
| CXCL1 | C-X-C motif ligand 1 |
| ER | Endoplasmic reticulum |
| HMOX | Heme oxygenase |
| HSP27 | Heat shock protein 27 |
| iNOS | Nitric oxide synthase |
| MBMDc | Murine bone-marrow derived cells |
| MCP1 | Monocyte chemoattractant protein |
| MDM | Monocyte-derived macrophages |
| NET | Neutrophil extracellular traps |
| NETosis | Neutrophil extracellular traps activation and release |
| NOS2 | Nitric oxide synthase |
| PBMCs | Peripheral blood mononuclear cells |
| PMA | Phorbol-12-myristate-13-acetate |
| SOD1 | Superoxide dismutase 1 |
| STC2 | Stanniocalcin-2 |
| TAM | Tumor-associated macrophages |
| TGFβ | Transforming growth factor beta |
| TNFα | Tumor necrosis factor alpha |
| VBN | Venous blood neutrophils |
| AD | Atopic dermatitis |
| BMDC | Bone marrow-derived dendritic cells |
| CCL17 | Chemokine (C-C motif) ligand 17 |
| CRT | Calreticulin |
| DCs | Dendritic cells |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HDM | House dust mite |
| HMGB1 | High mobility group protein B1 |
| HSP70 | Heat shock protein 70 |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| MHC II | Major histocompatibility complex class II |
| NF-κB | Nuclear factor kappa B |
| NK cells | Natural killer cells |
| PBS | Phosphate-buffered saline |
| TSLP | Thymic stromal lymphopoietin |
References
- Keevil, S.F. Physics and medicine: A historical perspective. Lancet 2012, 379, 1517–1524. [Google Scholar] [CrossRef]
- Melzer, A.; Cochran, S.; Prentice, P.; MacDonald, M.P.; Wang, Z.; Cuschieri, A. The importance of physics to progress in medical treatment. Lancet 2012, 379, 1534–1543. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Naidis, G.V. Modeling of plasmas for biomedicine. Trends Biotechnol. 2018, 36, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for treating cancer: Opportunities for adaptive and self-adaptive approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Szili, E.J.; Hong, S.H.; Oh, J.S.; Gaur, N.; Short, R.D. Tracking the penetration of plasma reactive species in tissue models. Trends Biotechnol. 2018, 36, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.J.; Huh, J.B.; Yun, M.J.; Chang, B.M.; Jeong, C.M.; Jeon, Y.C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont. 2013, 5, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R. Nonthermal plasma sterilization of living and nonliving surfaces. Annu. Rev. Biomed. Eng. 2012, 14, 255–274. [Google Scholar] [CrossRef]
- Kubinova, S.; Zaviskova, K.; Uherkova, L.; Zablotskii, V.; Churpita, O.; Lunov, O.; Dejneka, A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017, 7, 45183. [Google Scholar] [CrossRef] [Green Version]
- Chatraie, M.; Torkaman, G.; Khani, M.; Salehi, H.; Shokri, B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci. Rep. 2018, 8, 5621. [Google Scholar] [CrossRef]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold plasmas for biofilm control: Opportunities and challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Smolkova, B.; Uzhytchak, M.; Lynnyk, A.; Kubinova, S.; Dejneka, A.; Lunov, O. A critical review on selected external physical cues and modulation of cell behavior: Magnetic nanoparticles, non-thermal plasma and lasers. J. Funct. Biomater. 2018, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jager, A.; Polivka, L.; Sykova, E.; Dejneka, A.; Kubinova, S. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Churpita, O.; Zablotskii, V.; Deyneka, I.G.; Meshkovskii, I.K.; Jager, A.; Sykova, E.; Kubinova, S.; Dejneka, A. Non-thermal plasma mills bacteria: Scanning electron microscopy observations. Appl. Phys. Lett. 2015, 106, 053703. [Google Scholar] [CrossRef]
- Rupf, S.; Lehmann, A.; Hannig, M.; Schafer, B.; Schubert, A.; Feldmann, U.; Schindler, A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010, 59, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Gweon, B.; Kim, H.; Kim, K.; Kim, M.; Shim, E.; Kim, S.; Choe, W.; Shin, J.H. Suppression of angiogenesis by atmospheric pressure plasma in human aortic endothelial cells. Appl. Phys. Lett. 2014, 104, 133701. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Ressel, V.; Freund, E.; Gelbrich, N.; Mustea, A.; Stope, M.B. Gas plasma-treated prostate cancer cells augment myeloid cell activity and cytotoxicity. Antioxidants 2020, 9, 323. [Google Scholar] [CrossRef]
- Wolff, C.M.; Kolb, J.F.; Weltmann, K.D.; von Woedtke, T.; Bekeschus, S. Combination treatment with cold physical plasma and pulsed electric fields augments ROS production and cytotoxicity in lymphoma. Cancers 2020, 12, 845. [Google Scholar] [CrossRef] [Green Version]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent advances in the application of cold plasma technology in foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef]
- Lopez, M.; Calvo, T.; Prieto, M.; Mugica-Vidal, R.; Muro-Fraguas, I.; Alba-Elias, F.; Alvarez-Ordonez, A. A review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, K.P.; Friedman, G.; Fridman, A.; Clyne, A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface 2012, 9, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.D.; von Woedtke, T. The plasma jet kINPen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T.; et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef]
- Daeschlein, G.; Napp, M.; Lutze, S.; Arnold, A.; von Podewils, S.; Guembel, D.; Junger, M. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. J. Dtsch. Dermatol. Ges. 2015, 13, 143–150. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Metelmann, H.R.; Weltmann, K.D. Clinical plasma medicine: State and perspectives of in vivo application of cold atmospheric plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O. Plasma will. Br. J. Dermatol. 2016, 174, 486–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, M.; Evans, M.M.; Ostrikov, K.K. Effect of atmospheric gas plasmas on cancer cell signaling. Int. J. Cancer 2014, 134, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Hong, Y.J.; Lim, J.; Choi, J.S.; Choi, E.H.; Kang, S.; Rhim, H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials 2018, 156, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from physical plasmas: Redox chemistry for biomedical therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolkova, B.; Lunova, M.; Lynnyk, A.; Uzhytchak, M.; Churpita, O.; Jirsa, M.; Kubinova, S.; Lunov, O.; Dejneka, A. Non-thermal plasma, as a new physicochemical source, to induce redox imbalance and subsequent cell death in liver cancer cell lines. Cell. Physiol. Biochem. 2019, 52, 119–140. [Google Scholar]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef]
- Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002, 3, 1129–1134. [Google Scholar] [CrossRef]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant. Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Niessner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical gas plasma jet technology targets murine melanoma in an immunogenic fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.G.; Xiang, B.; Merlino, D.J.; Baybutt, T.R.; Sahu, J.; Fridman, A.; Snook, A.E.; Miller, V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology 2018, 7, e1484978. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemiere, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-thermal plasma as a unique delivery system of short-lived reactive oxygen and nitrogen species for immunogenic cell death in melanoma cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, D.; Costas, R.; Lariviere, V. Misconduct policies, academic culture and career stage, not gender or pressures to publish, affect scientific integrity. PLoS ONE 2015, 10, e0127556. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.J. Time to do something about reproducibility. eLife 2014, 3, e03981. [Google Scholar] [CrossRef]
- Stern, A.M.; Casadevall, A.; Steen, R.G.; Fang, F.C. Financial costs and personal consequences of research misconduct resulting in retracted publications. eLife 2014, 3, e02956. [Google Scholar] [CrossRef]
- Mechanism matters. Nat. Med. 2010, 16, 347. [CrossRef]
- Ehrenstein, M.R.; Mauri, C. If the treatment works, do we need to know why?: The promise of immunotherapy for experimental medicine. J. Exp. Med. 2007, 204, 2249–2252. [Google Scholar] [CrossRef] [Green Version]
- Isbary, G.; Shimizu, T.; Li, Y.F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold atmospheric plasma devices for medical issues. Expert Rev. Med. Devices 2013, 10, 367–377. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012, 21, 034005. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef] [Green Version]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Al-Abduly, A.; Christensen, P. An in situ and downstream study of non-thermal plasma chemistry in an air fed dielectric barrier discharge (DBD). Plasma Sources Sci. Technol. 2015, 24, 065006. [Google Scholar] [CrossRef] [Green Version]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef]
- Rumbach, P.; Witzke, M.; Sankaran, R.M.; Go, D.B. Decoupling interfacial reactions between plasmas and liquids: Charge transfer vs. plasma neutral reactions. J. Am. Chem. Soc. 2013, 135, 16264–16267. [Google Scholar] [CrossRef]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [Green Version]
- Shiraiwa, M.; Sosedova, Y.; Rouviere, A.; Yang, H.; Zhang, Y.; Abbatt, J.P.; Ammann, M.; Poschl, U. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nat. Chem. 2011, 3, 291–295. [Google Scholar] [CrossRef]
- Endre, J.S.; James, W.B.; Robert, D.S. A ‘tissue model’ to study the plasma delivery of reactive oxygen species. J. Phys. D Appl. Phys. 2014, 47, 152002. [Google Scholar]
- Lackmann, J.W.; Wende, K.; Verlackt, C.; Golda, J.; Volzke, J.; Kogelheide, F.; Held, J.; Bekeschus, S.; Bogaerts, A.; Schulz-von der Gathen, V.; et al. Chemical fingerprints of cold physical plasmas—An experimental and computational study using cysteine as tracer compound. Sci. Rep. 2018, 8, 7736. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [Green Version]
- Gweon, B.; Kim, D.; Kim, D.B.; Jung, H.; Choe, W.; Shin, J.H. Plasma effects on subcellular structures. Appl. Phys. Lett. 2010, 96, 101501. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, W.; Kim, K.T.; Lee, J.K. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl. Phys. Lett. 2010, 96, 021502. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [Green Version]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Lunova, M.; Jirsa, M.; Dejneka, A.; Kubinova, S. Chemically different non-thermal plasmas target distinct cell death pathways. Sci. Rep. 2017, 7, 600. [Google Scholar] [CrossRef] [Green Version]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jaeger, A.; Polivka, L.; Sykova, E.; Terebova, N.; Kulikov, A.; Kubinova, S.; Dejneka, A. Towards the understanding of non-thermal air plasma action: Effects on bacteria and fibroblasts. RSC Adv. 2016, 6, 25286–25292. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.; Choe, W.; Yong, H.I.; Jo, C.; Kim, K. Plasma-functionalized solution: A potent antimicrobial agent for biomedical applications from antibacterial therapeutics to biomaterial surface engineering. ACS Appl. Mater. Interfaces 2017, 9, 43470–43477. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Muller, K.; Dombrowski, F.; von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side effects by oral application of atmospheric pressure plasma on the mucosa in mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.; Rutkowski, R.; Hauschild, A.; Shojaei, R.K.; von Woedtke, T.; Rana, A.; Bauer, G.; Metelmann, P.; Seebauer, C. Side effects in cold plasma treatment of advanced oral cancer-clinical data and biological interpretation. Clin. Plasma Med. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Coleman, J.J.; Ferner, R.E.; Evans, S.J. Monitoring for adverse drug reactions. Br. J. Clin. Pharmacol. 2006, 61, 371–378. [Google Scholar] [CrossRef]
- Coleman, J.J.; Pontefract, S.K. Adverse drug reactions. Clin. Med. 2016, 16, 481–485. [Google Scholar] [CrossRef]
- McDowell, S.E.; Thomas, S.K.; Coleman, J.J.; Aronson, J.K.; Ferner, R.E. A practical guide to monitoring for adverse drug reactions during antihypertensive drug therapy. J. R. Soc. Med. 2013, 106, 87–95. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA-a review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [Green Version]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. 2016, 798–799, 48–54. [Google Scholar] [CrossRef]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef] [Green Version]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Temkin, V.; Karin, M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: The receptor-interacting protein 1 odyssey. Immunol. Rev. 2007, 220, 8–21. [Google Scholar] [CrossRef]
- Jin, J.; Sklar, G.E.; Min Sen Oh, V.; Chuen Li, S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008, 4, 269–286. [Google Scholar]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hugel, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef]
- Emmert, S.; Brehmer, F.; Hänßle, H.; Helmke, A.; Mertens, N.; Ahmed, R.; Simon, D.; Wandke, D.; Maus-Friedrichs, W.; Däschlein, G.; et al. Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clin. Plasma Med. 2013, 1, 24–29. [Google Scholar] [CrossRef]
- Isbary, G.; Shimizu, T.; Zimmermann, J.L.; Heinlin, J.; Al-Zaabi, S.; Rechfeld, M.; Morfill, G.E.; Karrer, S.; Stolz, W. Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clin. Plasma Med. 2014, 2, 50–55. [Google Scholar] [CrossRef]
- Klebes, M.; Lademann, J.; Philipp, S.; Ulrich, C.; Patzelt, A.; Ulmer, M.; Kluschke, F.; Kramer, A.; Weltmann, K.D.; Sterry, W.; et al. Effects of tissue-tolerable plasma on psoriasis vulgaris treatment compared to conventional local treatment: A pilot study. Clin. Plasma Med. 2014, 2, 22–27. [Google Scholar] [CrossRef]
- Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Li, Y.F.; Morfill, G.E.; Thomas, H.M.; Steffes, B.; Heinlin, J.; Karrer, S.; Stolz, W. Non-thermal plasma—More than five years of clinical experience. Clin. Plasma Med. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Dickersin, K.; Straus, S.E.; Bero, L.A. Evidence based medicine: Increasing, not dictating, choice. BMJ 2007, 334 (Suppl. 1), s10. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, T. The importance of applying evidence-based medicine in clinical practice. In Management of Hypertension: Current Practice and the Application of Landmark Trials; Papademetriou, V., Andreadis, E.A., Geladari, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Guyatt, G.H. Progress in evidence-based medicine: A quarter century on. Lancet 2017, 390, 415–423. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y. Evidence based medicine: Does it make a difference? Numerophobia may be a problem in adopting evidence based medicine. BMJ 2005, 330, 93. [Google Scholar]
- Rosenberg, W.; Donald, A. Evidence based medicine: An approach to clinical problem-solving. BMJ 1995, 310, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Sabri, A.A.; Qayyum, M.A. The problem of evidence-based medicine in developing countries. CMAJ 2006, 175, 62. [Google Scholar] [CrossRef] [Green Version]
- Woolf, S.H.; Grol, R.; Hutchinson, A.; Eccles, M.; Grimshaw, J. Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ 1999, 318, 527–530. [Google Scholar] [CrossRef]
- Kredo, T.; Bernhardsson, S.; Machingaidze, S.; Young, T.; Louw, Q.; Ochodo, E.; Grimmer, K. Guide to clinical practice guidelines: The current state of play. Int. J. Qual. Health Care 2016, 28, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.M.; Peterson, P.N.; Masoudi, F.A. Evaluating the evidence—Is there a rigid hierarchy? Circulation 2008, 118, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Yetley, E.A.; MacFarlane, A.J.; Greene-Finestone, L.S.; Garza, C.; Ard, J.D.; Atkinson, S.A.; Bier, D.M.; Carriquiry, A.L.; Harlan, W.R.; Hattis, D.; et al. Options for basing Dietary Reference Intakes (DRIs) on chronic disease endpoints: Report from a joint US-/Canadian-sponsored working group. Am. J. Clin. Nutr. 2017, 105, 249S–285S. [Google Scholar] [CrossRef] [Green Version]
- Atkins, D.; Briss, P.A.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Hill, S.; Jaeschke, R.; Liberati, A.; Magrini, N.; et al. Systems for grading the quality of evidence and the strength of recommendations II: Pilot study of a new system. BMC Health Serv. Res. 2005, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. BMJ 2001, 323, 334–336. [Google Scholar] [CrossRef] [Green Version]
- Haidich, A.B. Meta-analysis in medical research. Hippokratia 2010, 14, 29–37. [Google Scholar]
- Hartling, L.; Dryden, D.M.; Guthrie, A.; Muise, M.; Vandermeer, B.; Donovan, L. Benefits and harms of treating gestational diabetes mellitus: A systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann. Intern. Med. 2013, 159, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Mbuagbaw, L.; Wiysonge, C.S.; Nsagha, D.S.; Ongolo-Zogo, P.; Pantoja, T. An introduction to systematic reviews and meta-analysis: A workshop report on promoting evidence based medical practice through capacity building in research synthesis. Pan Afr. Med. J. 2011, 8, 15. [Google Scholar] [CrossRef]
- O’Rourke, K.; Detsky, A.S. Meta-analysis in medical research: Strong encouragement for higher quality in individual research efforts. J. Clin. Epidemiol. 1989, 42, 1021–1024. [Google Scholar] [CrossRef]
- Wende, K.; von Woedtke, T.; Weltmann, K.D.; Bekeschus, S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biol. Chem. 2018, 400, 19–38. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Baran, C.P.; Zeigler, M.M.; Tridandapani, S.; Marsh, C.B. The role of ROS and RNS in regulating life and death of blood monocytes. Curr. Pharm. Des. 2004, 10, 855–866. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic macrophage-released tumour necrosis factor-alpha (TNF-alpha) as a killing mechanism for cancer cell death after cold plasma activation. J. Phys. D Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Freund, E.; Moritz, J.; Stope, M.; Seebauer, C.; Schmidt, A.; Bekeschus, S. Plasma-derived reactive species shape a differentiation profile in human monocytes. Appl. Sci. 2019, 9, 2530. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Scherwietes, L.; Freund, E.; Liedtke, K.R.; Hackbarth, C.; von Woedtke, T.; Partecke, L.I. Plasma-treated medium tunes the inflammatory profile in murine bone marrow-derived macrophages. Clin. Plasma Med. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Bethge, L.; Masur, K.; von Woedtke, T.; Hasse, S.; Wende, K. Redox stimulation of human THP-1 monocytes in response to cold physical plasma. Oxid. Med. Cell. Longev. 2016, 2016, 5910695. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Rodder, K.; Hasse, S.; Masur, K.; Toups, L.; Lillig, C.H.; von Woedtke, T.; Wende, K.; Bekeschus, S. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Process. Polym. 2016, 13, 1179–1188. [Google Scholar] [CrossRef]
- Kaushik, N.; Lee, S.J.; Choi, T.G.; Baik, K.Y.; Uhm, H.S.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells. Sci. Rep. 2015, 5, 8726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekeschus, S.; Winterbourn, C.C.; Kolata, J.; Masur, K.; Hasse, S.; Broker, B.M.; Parker, H.A. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J. Leukocyte Biol. 2016, 100, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-pulsed DBD plasma-generated reactive oxygen species trigger immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Truong, B.; Pappas, A.; Kirifides, L.; Oubarri, A.; Chen, S.; Lin, S.; Dobrynin, D.; Fridman, G.; Fridman, A.; et al. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma Process. Polym. 2015, 12, 1392–1399. [Google Scholar] [CrossRef]
- Bundscherer, L.; Wende, K.; Ottmuller, K.; Barton, A.; Schmidt, A.; Bekeschus, S.; Hasse, S.; Weltmann, K.D.; Masur, K.; Lindequist, U. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology 2013, 218, 1248–1255. [Google Scholar] [CrossRef]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold atmospheric plasma induces apoptosis and oxidative stress pathway regulation in T-lymphoblastoid leukemia cells. Oxid. Med. Cell. Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schütz, C.S.; Nießner, F.; Wende, K.; Weltmann, K.-D.; Gelbrich, N.; von Woedtke, T.; Schmidt, A.; Stope, M.B. Elevated H2AX phosphorylation observed with kINPen plasma treatment is not caused by ROS-mediated DNA damage but is the consequence of apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 8535163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crestale, L.; Laurita, R.; Liguori, A.; Stancampiano, A.; Talmon, M.; Bisag, A.; Gherardi, M.; Amoruso, A.; Colombo, V.; Fresu, L.G. Cold atmospheric pressure plasma treatment modulates human monocytes/macrophages responsiveness. Plasma 2018, 1, 261–276. [Google Scholar] [CrossRef] [Green Version]
- Rodder, K.; Moritz, J.; Miller, V.; Weltmann, K.D.; Metelmann, H.R.; Gandhirajan, R.; Bekeschus, S. Activation of murine immune cells upon co-culture with plasma-treated B16F10 melanoma cells. Appl. Sci. 2019, 9, 660. [Google Scholar] [CrossRef] [Green Version]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Bundscherer, L.; Bekeschus, S.; Tresp, H.; Hasse, S.; Reuter, S.; Weltmann, K.-D.; Lindequist, U.; Masur, K. Viability of human blood leukocytes compared with their respective cell lines after plasma treatment. Plasma Med. 2013, 3, 71–80. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Flieswasser, T.; Freire Boullosa, L.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold atmospheric plasma-treated PBS eliminates immunosuppressive pancreatic stellate cells and induces immunogenic cell death of pancreatic cancer cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef] [Green Version]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014, 3, e955691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedtke, K.R.; Freund, E.; Hackbarth, C.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clin. Plasma Med. 2018, 11, 10–17. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Yonetamari, K.; Shirakawa, Y.; Akiyama, T.; Ono, R. Anti-tumor immune response induced by nanosecond pulsed streamer discharge in mice. J. Phys. D Appl. Phys. 2017, 50, 12LT01. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, M.H.; Kim, H.J.; Won, H.R.; Kim, C.H. Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression. Sci. Rep. 2017, 7, 15564. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, Y.S.; Kim, H.J.; Han, C.H.; Kang, S.U.; Kim, C.H. Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice. Sci. Rep. 2019, 9, 13510. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why most published research findings are false. PLoS Med. 2005, 2, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.A. Why most published research findings are false: Author’s reply to Goodman and Greenland. PLoS Med. 2007, 4, 1132–1133. [Google Scholar] [CrossRef] [Green Version]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.C.; Steen, R.G.; Casadevall, A. Misconduct accounts for the majority of retracted scientific publications. Proc. Natl. Acad. Sci. USA 2012, 109, 17028–17033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R. Rigor Mortis: How Sloppy Science Creates Worthless Cures, Crushes Hope, and Wastes Billions, 1st ed.; Basic Books: New York, NY, USA, 2017; p. 288. [Google Scholar]
- Begley, C.G.; Ellis, L.M. Drug development: Raise standards for preclinical cancer research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef]
- Freedman, L.P.; Cockburn, I.M.; Simcoe, T.S. The economics of reproducibility in preclinical research. PLoS Biol. 2015, 13, e1002165. [Google Scholar] [CrossRef] [PubMed]
- Hines, W.C.; Su, Y.; Kuhn, I.; Polyak, K.; Bissell, M.J. Sorting out the FACS: A devil in the details. Cell Rep. 2014, 6, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Abrink, M.; Gobl, A.E.; Huang, R.; Nilsson, K.; Hellman, L. Human cell lines U-937, THP-1 and Mono Mac 6 represent relatively immature cells of the monocyte-macrophage cell lineage. Leukemia 1994, 8, 1579–1584. [Google Scholar] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008, 2, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 1996, 59, 555–561. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Kohro, T.; Tanaka, T.; Murakami, T.; Wada, Y.; Aburatani, H.; Hamakubo, T.; Kodama, T. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J. Atheroscler. Thromb. 2004, 11, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Prinz, F.; Schlange, T.; Asadullah, K. Believe it or not: How much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov. 2011, 10, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinney, D.C. Biochemical mechanisms of drug action: What does it take for success? Nat. Rev. Drug Discov. 2004, 3, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Chernajovsky, Y. The importance of understanding the molecular mechanisms of resistance to biologics. Rheumatology 2012, 51, 397–398. [Google Scholar] [CrossRef] [Green Version]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| ROS or RNS Name | Chemical Formula |
|---|---|
| Superoxide anion | O2− |
| Hydrogen peroxide | H2O2 |
| Hydroxyl radical | •OH |
| Singlet oxygen | 1O2 |
| Ozone | O3 |
| Organic radicals | RO•, RO2• |
| Nitric oxide | •NO |
| Nitrogen dioxide | •NO2 |
| Peroxynitrite | ONOO− |
| Plasma Type | Pathological Condition | Side Effects | Type of Study | Ref. |
|---|---|---|---|---|
| APPJ | Chronic leg ulcers | No signs of cytotoxicity | Cohort study | [91] |
| DBD | Skin infection eczema | No side effects | Case study | [92] |
| APPJ | Chronic infected skin wounds | Pain (before and after treatment) | Clinical trial | [25] |
| APPJ | Skin ulcers | No side effects | Case study | [23] |
| APPJ | Head and neck cancer | Bad taste, pain, collateral edema, bleeding, sialorrhea, necrosis | Case control study | [78] |
| APPJ | Skin herpes zoster | No side effects | Clinical trial | [93] |
| APPJ | Skin psoriasis vulgaris | No side effects | Case study | [94] |
| APPJ | Skin chronic wounds | No side effects | Case control study | [95] |
| APPJ | Skin wounds | Focal mucosal erosion with superficial ulceration and necrosis accompanied by a mild inflammatory reaction. | Animal study | [79] |
| Plasma Device | Physicochemical Parameters | Cell Lineage | Signaling Pathway | Main Results | Ref. | ||
|---|---|---|---|---|---|---|---|
| Gas | Voltage (kV) | Frequency | |||||
| kiNPen 11 | Ar | N.A. | ~1 MHz | THP-1 | Inflammation | ↑IL-8 mRNA level and secretion; | [124] |
| ↑HMOX mRNA level | |||||||
| kINPen 09 | Ar | 2–6 | ~1 MHz | Jurkat and THP-1 | Jurkat cells apoptosis, | ↑resistance of THP-1 to plasma-treated medium in comparison to Jurkat cells; | [125] |
| THP-1 anti-oxidant defense | differences in expression levels of genes involved in redox and anti-oxidant system regulation and apoptosis. | ||||||
| APPJ | Air | 2 | N.A. | THP-1, U937 and RAW264.7, PBMCs | Apoptosis | Inhibition of cell growth; | [126] |
| ↓Glucose consumption, | |||||||
| intracellular ATP and lactic acid production; | |||||||
| mitochondria membrane depolarization, | |||||||
| cytochrome c release and induction of apoptosis. | |||||||
| DBD | N2 | 1.08 | 30 kHz | T98G and A549 in co-culture | Macrophage activation, cancer cells death induction | ↑expression of iNOS and TNF-α genes on mRNA and protein levels; | [121] |
| with RAW264.7 | plasma-activated macrophages induced the cell death of glioma and adenocarcinoma in co-culture | ||||||
| kINPen 11 | Ar | N.A. | 1 MHz | Neutrophils isolated from | NETosis | Activation of NETosis in neutrophils; Release of DNA, extracellular DNA. | [127] |
| venous blood | |||||||
| kINPen | Ar | N.A. | N.A. | THP-1, A375, primary monocytes | Alternation in metabolic activity | Altered the morphology of THP1 cells; changes in surface markers expression; ↑IL8 and MCP-1 in PMA-stimulated THP-1 ↑ IL1β, IL6, and IL8 | [122] |
| isolated from PBMCs | and morphology | ↑HLA-DR (an M1 macrophage marker) and fibronectin (and M2 macrophage marker) | |||||
| DBD | Air | 29 | 15 and 30 Hz | THP-1, A549 in co-culture | ICD | induction of ICD in A549 cells | [128] |
| (↑calreticulin, ROS production, ATP secretion); | |||||||
| ↓viability of Plasma treated A549 cells, | |||||||
| when co-cultured with M0 macrophages | |||||||
| DBD | N.A. | 29 | 5, 15, 30, 75 Hz | CNE-1, THP-1 | ER stress, ICD | ↑ immunogenic cell death of cancer cells; | [129] |
| ↑ATP secretion; | |||||||
| ↑ER stress proteins (↑ATF4-STC2 pathway). | |||||||
| kiNPen | Ar | 2–6 | 1.1 MHz | Jurkat, THP-1 | Apoptosis | ↑resistance of THP-1 cells to plasma treatment in comparison with Jurkat cells, | [130] |
| ↑ caspase 3 dependent apoptosis; | |||||||
| ↑ERK 1/2 and MEK 1/2 and p38 MAPK and JNK 1/2; | |||||||
| ↑HSP27 in THP-1.t | |||||||
| kiNPen | Ar | N.A. | 1 MHz | Jurkat, U-937 | Apoptosis, Ferroptosis | Plasma treatment in combination with pulsed electric fields (electro square porator) | [19] |
| resulted in ↑cytotoxicity in Jurkat cells. Contrary, the additive effect was smaller in U937 cells; | |||||||
| activation of apoptosis; | |||||||
| ↑ROS production, caspase 3/7 activation). | |||||||
| DBD | Air | 20 | 500 Hz | Jurkat | Apoptosis | ↑p53 protein, but not on mRNA level 48 h post plasma treatment; | [131] |
| ↑Bax and Bcl-2 proteins after 24 h, slightly ↑caspase-8; | |||||||
| ↑mRNA levels of antioxidant enzyme SOD1, CAT, and GSR2 6 and 24 h post NTP treatment | |||||||
| as a response to ROS elevated oxidative stress | |||||||
| kINPen | Ar | N.A. | N.A. | TK6 | DNA damage response | ↑γH2AX post plasma treatment as a consequence of ROS induced | [132] |
| oxidative stress in apoptosis | |||||||
| DBD | Air | 25 | 20 kHz | Human monocytes isolated from venous blood, MDM | ROS production, surface markers expression | ↓CD86, CD36, CD163 and CD206; | [133] |
| ↓CD16 post NTP treatment; | |||||||
| NTP treatment of MDM led to time-dependent ↓M1 population, significantly after 30 sec of treatment, following ↑M2 population. | |||||||
| kINPen MED | Ar | N.A. | N.A. | MBMDc, PDA6606 in co-culture | Macrophage polarization | ↑NOS2 in TAM; | [123] |
| slight ↑M2 polarized macrophages post exposure with plasma- treated medium; | |||||||
| ↑CXCL1 and CCL4 in non-polarized macrophages post plasma-treated medium; | |||||||
| ↓CXCL1, CCL4, MCP1 in TAM. | |||||||
| kINPen | Ar | 2–6 | 1 MHz | splenocytes of mice spleens, B16F10 in co-culture | Immune cells activation | ↓metabolic activity in naive and PMA-stimulated splenocytes; | [134] |
| ↑IL-10, CCL4, IL-4, IL-12, and IL-1β in naive splenocytes; | |||||||
| ↑calcium influx in splenocytic T-cells, but not in macrophages; | |||||||
| Co-culturing of monocytes with plasma-treated melanoma cells ↑CD115, IL-10 and CCL4, with a slightly ↑IL-1β, IL-12p70, TNFα, and TGFβ. | |||||||
| Co-culture of CD4+ T helper and CD8+ cytotoxic T cells with plasma-treated melanoma cells showed an increase of CD4 over CD8 cells (↑CD28). | |||||||
| Plasma Device | Physicochemical Parameters | Animal Model | Signaling Pathway | Main Results | Ref. | ||
|---|---|---|---|---|---|---|---|
| Gas | Voltage (kV) | Frequency | |||||
| kINPen MED | Ar | N.A. | N.A. | C57BL/6 mice | Immuno-modulation | ↓total number of tumor nodes; | [144] |
| ↑infiltration of macrophages, but not CD206+ cells into tumors; | |||||||
| ↑ number of macrophages and T cells, | |||||||
| with no changes in numbers of dendritic cells and neutrophils. Increased level of calreticulin | |||||||
| kINPen MED | Ar | N.A. | 1 MHz | C57BL/6 mice | Apoptosis in tumor tissue | Induction of apoptosis in tumor tissues; | [145] |
| No significant differences in the number of granulocytes, monocytes, and lymphocytes in general; | |||||||
| No changes in cytokines secretion of IL6, IL10, IL12, MCP1, IFNγ, or TNFα. | |||||||
| APPJ | O2 or N2 | 24 | N.A. | CD2F1 and C57BL/6 mice | Tumor growth inhibition | ↓tumor size in CD2F1 mice; | [146] |
| ↑IFN-γ, no changes in TNF-α from splenocytes of the plasma-treated CD2F1 mice; | |||||||
| In the C57BL/6 mice very weak response to plasma-treatment; | |||||||
| Discussion on immune response, but no data are provide to | |||||||
| support it. | |||||||
| kINPen | Ar | N.A. | N.A. | Balb/C mice | ICD | ↑immunogenic cell death markers in CT-26 cells; | [96] |
| heat shock protein 70 (HSP70), and high-mobility-group-protein B1 (HMGB1); | |||||||
| ↑IL1β, IL6, IL12p70, CCL4, and TNFα. | |||||||
| ↑number of macrophages and T cells in mice | |||||||
| with CT26 peritoneal carcinomatosis post treatment with oxidized saline solution. | |||||||
| DBD | Air | 17 | 50–500 Hz | C57BL/6J mice | ICD | Activation of immunogenic cell death marker (calreticulin); | [48] |
| ↑survival rate of mice post vaccine injection prepared from B16F10 melanoma cells treated with DBD plasma. | |||||||
| kINPen | Ar, Ar+O2, He, He+O2 | N.A. | 1 MHz | C57BL/6 mice | ICD | ↓tumor growth | [46] |
| ↑CD8+ cytotoxic T-cells; | |||||||
| ↑macrophages; | |||||||
| ↑CD11c+ dendritic cells (DCs); | |||||||
| ↑CD127 in both CD4+ and CD8+ T-cells; | |||||||
| ↑ICD markers in B16F10 (↑CRT, HSP90, CD47); | |||||||
| Co-culture of splenocytes isolated from vaccinated mice with B16F10 ↑marker CD69 in CD8+ T cells and ↑CXCL1, CXCL10, IFNγ, IL1α, IL6, and TNFα; | |||||||
| ↓GM-CSF, CCL17. | |||||||
| APPJ | N2 | N.A. | N.A. | C57/BL6 mice | Anti-inflammatory effect | ↓immune cells infiltration (CD4+ T cells, CD11c+ cells, CD11b+ cells, and Gr-1+ cells); | [147] |
| ↓pro-inflammatory cytokine and chemokine (IL-6, IL-17, IL-22, CCL20 and CXCL1); | |||||||
| ↓Th17 cell differentiation in lymph node; | |||||||
| In vitro suppressed differentiation of naive CD4+T cells into Th17 cells and Th1 cells; | |||||||
| ↓CD80, CD86, and MHCII in BDCM and ↓IL-6 expression TNF-α and IL-6. | |||||||
| APPJ | N2 | 5 | 15 kHz | NC/Nga mice | Anti-inflammatory effect | In vivo: NTP treatment ↓HDM-induced infiltration of mast cells and eosinophil into the dermis and ↓Th2 cell differentiation; | [148] |
| ↓TSLP and CCL17 post NTP treatment in HDM-induced AD; | |||||||
| In vitro: Activated mast cells incubation in plasma- treated medium resulted in ↓NF-κB, TNF-α, IL-6 and IL-13 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolková, B.; Frtús, A.; Uzhytchak, M.; Lunova, M.; Kubinová, Š.; Dejneka, A.; Lunov, O. Critical Analysis of Non-Thermal Plasma-Driven Modulation of Immune Cells from Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 6226. https://doi.org/10.3390/ijms21176226
Smolková B, Frtús A, Uzhytchak M, Lunova M, Kubinová Š, Dejneka A, Lunov O. Critical Analysis of Non-Thermal Plasma-Driven Modulation of Immune Cells from Clinical Perspective. International Journal of Molecular Sciences. 2020; 21(17):6226. https://doi.org/10.3390/ijms21176226
Chicago/Turabian StyleSmolková, Barbora, Adam Frtús, Mariia Uzhytchak, Mariia Lunova, Šárka Kubinová, Alexandr Dejneka, and Oleg Lunov. 2020. "Critical Analysis of Non-Thermal Plasma-Driven Modulation of Immune Cells from Clinical Perspective" International Journal of Molecular Sciences 21, no. 17: 6226. https://doi.org/10.3390/ijms21176226