Investigation of Permeation of Theophylline through Skin Using Selected Piperazine-2,5-Diones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry and Physicochemical Properties
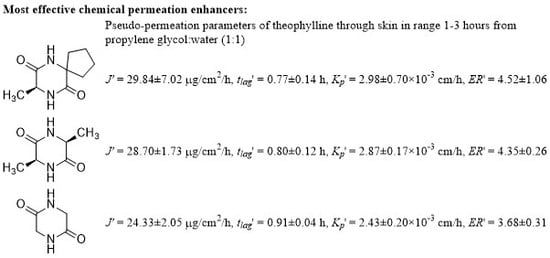
2.2. Permeation Experiments
2.3. In Vitro Cytotoxicity Assay
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.3. In Vitro Transdermal Permeation Experiments
3.4. In Vitro Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shargel, L.; Yu, A.B.C. Applied Biopharmaceutics and Pharmacokinetics, 7th ed.; McGraw-Hill: New York, NY, USA, 2016. [Google Scholar]
- Watkinson, A.C.; Kearney, M.C.; Quinn, H.L.; Courtenay, A.J.; Donnelly, R.F. Future of the transdermal drug delivery market—Have we barely touched the surface? Expert Opin. Drug Deliv. 2016, 13, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Forslind, B.; Lindberg, M. Skin, Hair, Nails: Structure and Function; Marcel & Dekker: New York, NY, USA, 2004. [Google Scholar]
- Cernikova, A.; Jampilek, J. Structure modification of drugs influencing their bioavailability and therapeutic effect. Chem. Listy 2014, 108, 7–16. [Google Scholar]
- Jampilek, J. Transdermal application of drugs and techniques affecting skin barrier. J. Bioequiv. Availab. 2013, 5, 233–235. [Google Scholar] [CrossRef]
- Jampilek, J.; Brychtova, K. Azone analogues: Classification, design, and transdermal penetration principles. Med. Res. Rev. 2012, 32, 907–947. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.K.; Saraogi, R. Natural products as potential drug permeation enhancer in transdermal drug delivery system. Arch. Dermatol. Res. 2014, 306, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Thong, H.Y.; Zhai, H.; Maibach, H.I. Percutaneous penetration enhancers: An overview. Skin Pharmacol. Physiol. 2007, 20, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Azone and its analogues. In Percutaneous Penetration Enhancers—Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Dragicevic-Curic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 69–106. [Google Scholar]
- Kasafirek, E.; Vanzura, J.; Krejci, I.; Krepelka, J.; Dlabac, A.; Valchar, M. 2,5-Piperazinedione Derivs. Belgian Patent 897843, 20 May 1984; Czechoslovakian Patent CS 231227, 26 January 1986. [Google Scholar]
- Radl, S.; Kasafirek, E.; Krejci, I. Alaptide. Drug. Future 1990, 15, 445–447. [Google Scholar] [CrossRef]
- Jampilek, J.; Opatrilova, R.; Coufalova, L.; Cernikova, A.; Dohnal, J. Utilization of Alaptide as Transdermal Penetration Modifier in Pharmaceutical Compositions for Human and Veterinary Applications Containing Anti-Inflammatory Drugs and/or Antimicrobial Chemotherapeutics. WO/2013/020527 A1, 14 February 2013. [Google Scholar]
- Jampilek, J.; Opatrilova, R.; Dvorakova, L.; Brychtova, K.; Dohnal, J. Utilization of Alaptide as Transdermal Penetration Modifier in Pharmaceutical Compositions for Human and Veterinary Applications Containing Non-Steroidal Anti-Inflammatory and/or Antipyretic-Analgesic Drugs. Czech Patent CZ 304915 B6, 10 December 2014. [Google Scholar]
- Jampilek, J.; Opatrilova, R.; Dvorakova, L.; Cernikova, A.; Dohnal, J. Utilization of Alaptide as Transdermal Penetration Modifier in Pharmaceutical Compositions for Human and Veterinary Applications Containing Antimicrobial Chemotherapeutics. Czech Patent CZ 306686 B6, 29 March 2017. [Google Scholar]
- Jampilek, J.; Opatrilova, R.; Dvorakova, L.; Dohnal, J. Utilization of Alaptide as Transdermal Penetration Modifier in Pharmaceutical Compositions for human and Veterinary Applications Containing Glucocorticoids. Czech Patent CZ 306770 B6, 17 May 2017. [Google Scholar]
- Jampilek, J.; Dohnal, J. Alaptide as transdermal permeation modifier. In Percutaneous Penetration Enhancers—Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Dragicevic-Curic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 115–132. [Google Scholar]
- Cernikova, A.; Bobal, P.; Bobalova, J.; Dohnal, J.; Jampilek, J. Investigation of permeation of acyclovir through skin using alaptide. Acta Chromatogr. 2018, 30, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Jampilek, J.; Opatrilova, R.; Rezacova, A.; Oktabec, Z.; Dohnal, J. Alaptide: Methods of Effecting Its Solubility, Membrane Permeation and Pharmaceutical Compositions for Human and/or Veterinary Applications. WO/2014/019556 A1, 6 February 2014. [Google Scholar]
- Kasafirek, E.; Rybak, M.; Krejci, I.; Sturs, A.; Krepela, E.; Sedo, A. Two-step generation of spirocyclic dipeptides from linear peptide ethyl ester precursors. Life Sci. 1992, 50, 187–193. [Google Scholar] [CrossRef]
- Vallejos, S.; Estevez, P.; Ibeas, S.; Munoz, A.; Garcia, F.C.; Serna, F.; Garcia, J.M. Selective and highly sensitive fluorescent probe of Hg2+ in organic and aqueous media: The role of a polymer network in extending the sensing phenomena to water environments. Sens. Actuators B Chem. 2011, 157, 686–690. [Google Scholar] [CrossRef]
- Chen, F.M.F.; Stainauer, R.; Benoiton, N.L. Mixed anhydrides in peptide synthesis. Reduction of urethane formation and racemization using N-methylpiperidine as the tertiary amine base. J. Org. Chem. 1983, 48, 2939–2941. [Google Scholar] [CrossRef]
- Pizova, H.; Bobal, P. Optimized and scalable synthesis of propylphosphonic anhydride for general use. Tetrahedron Lett. 2015, 56, 2014–2017. [Google Scholar] [CrossRef]
- Pizova, H.; Havelkova, M.; Stepankova, S.; Bak, A.; Kauerova, T.; Kozik, V.; Oravec, M.; Imramovsky, A.; Kollar, P.; Bobal, P.; et al. Proline-based carbamates as cholinesterase inhibitors. Molecules 2017, 22, 1969. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Smolinski, A.; Jampilek, J. Multidimensional (3D/4D-QSAR) probability-guided pharmacophore mapping: Investigation of activity profile for a series of drug absorption promoters. RSC Adv. 2016, 6, 76183–76205. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Smolinski, S.; Jampilek, J. In silico estimation of basic activity-relevant parameters for a set of drug absorption promoters. SAR QSAR Environ. Res. 2017, 28, 427–449. [Google Scholar] [CrossRef]
- Kapustikova, I.; Bak, A.; Gonec, T.; Kos, J.; Kozik, V.; Jampilek, J. Investigation of hydro-lipophilic properties of N-alkoxyphenylhydroxynaphthalenecarboxamides. Molecules 2018, 23, 1635. [Google Scholar] [CrossRef]
- Bąk, A.; Kozik, V.; Walczak, M.; Fraczyk, J.; Kaminski, Z.; Kolesinska, B.; Smolinski, A.; Jampilek, J. Towards intelligent drug design system: Application of artificial dipeptide receptor library in QSAR-oriented study. Molecules 2018, 23, 1964. [Google Scholar] [CrossRef]
- Malik, I.; Csollei, J.; Solovic, I.; Pospisilova, S.; Michnova, H.; Jampilek, J.; Cizek, A.; Kapustikova, I.; Curillova, J.; Pechacova, M.; et al. Dibasic derivatives of phenylcarbamic acid against mycobacterial strains: Old drugs and new tricks? Molecules 2018, 23, 2493. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Malik, I.; Jampilek, J.; Smolinski, S. Probability-driven 3D pharmacophore mapping of antimycobacterial potential of hybrid molecules combining phenylcarbamoyloxy and N-arylpiperazine fragments. SAR QSAR Environ. Res. 2018, 29, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Japertas, P.; Didziapetris, R.; Petrauskas, A. Fragmental methods in the analysis of biological activities of diverse compound sets. Mini-Rev. Med. Chem. 2003, 3, 797–808. [Google Scholar] [CrossRef] [PubMed]
- DeWitte, R.S. Understanding polyelectrolytes. Mod. Drug Discov. 2004, 7, 41–44. [Google Scholar]
- Franz, T.J. Percutaneous absorption. On the relevance of In vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Urea analogues in propylene glycol as penetration enhancers in human skin. Int. J. Pharm. 1989, 56, 43–50. [Google Scholar] [CrossRef]
- Yamane, M.A.; Williams, A.C.; Barry, B.W. Terpene penetration enhancers in propylene glycol/water co-solvent systems: Effectiveness and mechanism of action. J. Pharm. Pharmacol. 1995, 47, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; El-Sayed, M.M.; Sanghvi, T.; Yalkowsky, S.H. Estimation of the effect of NaCl on the solubility of organic compounds in aqueous solutions. J. Pharm. Sci. 2000, 89, 1620–1625. [Google Scholar] [CrossRef]
- Katz, M.; Ben-Shlush, I.; Kolusheva, S.; Jelinek, R. Rapid colorimetric screening of drug interaction and penetration through lipid barriers. Pharm. Res. 2006, 23, 580–588. [Google Scholar] [CrossRef]
- Fang, J.Y.; Tsai, T.H.; Hung, C.F.; Wong, W.W. Development and evaluation of the essential oil from Magnolia fargesii for enhancing the transdermal absorption of theophylline and cianidanol. J. Pharm. Pharmacol. 2004, 56, 1493–1500. [Google Scholar] [CrossRef]
- Sloan, K.B.; Beall, H.D.; Taylor, H.E.; Getz, J.J.; Villaneuva, R.; Nipper, R.; Smith, K. Transdermal delivery of theophylline from alcohol vehicles. Int. J. Pharm. 1998, 171, 185–193. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An In vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Schwarz, K.; Neurand, K.T. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr. Probl. Dermatol. 1978, 7, 39–52. [Google Scholar] [PubMed]
- Suffness, M.; Douros, J. Current status of the NCI plant and animal product program. J. Nat. Prod. 1982, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Beagle, L.K.; Hansen, F.K.; Monbaliu, J.C.M.; DesRosiers, M.P.; Phillips, A.M.; Stevens, C.V.; Katritzky, A.R. Efficient Synthesis of 2,5-diketopiperazines by Staudinger-mediated cyclization. Synlett 2012, 23, 2337–2340. [Google Scholar]
- Ishibashi, N.; Kouge, K.; Shinoda, I.; Kanehisa, H.; Okai, H. Studies on flavored peptides. Part V. A mechanism for bitter taste sensibility in peptides. Agric. Biol. Chem. 1988, 52, 819–827. [Google Scholar]
- Chu, D.T.W.; Nordeen, C.W.; Hardy, D.J.; Swanson, R.N.; Giardina, W.J.; Pernet, A.G.; Plattner, J.J. Synthesis, antibacterial activities, and pharmacological properties of enantiomers of temafloxacin hydrochloride. J. Med. Chem. 1991, 34, 168–174. [Google Scholar] [CrossRef]
- Campbell, T.D.; Hart, C.A.; Febrian, R.; Cheneler, M.L.; Bracher, P.J. The opposite effect of K+ and Na+ on the hydrolysis of linear and cyclic dipeptides. Tetrahedron Lett. 2018, 59, 2264–2267. [Google Scholar] [CrossRef]
- Woodard, R.W. Stereochemistry of cyclic dipeptides. Assignment of the prochiral methylenes of 1-aminocyclopropane-1-carboxylic acid. J. Org. Chem. 1985, 50, 4796–4799. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Section 4, Test No. 428: Skin Absorption: In Vitro Method; OECD Publishing: Paris, France, 2004.
- WHO. Environmental Health Criteria (EHC 235)—Dermal Absorption; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Shah, J.C. Application of kinetic model to In vitro percutaneous permeation of drugs. Int. J. Pharm. 1996, 133, 179–189. [Google Scholar] [CrossRef]
- Steffansen, B.; Brodin, B.; Uhd Nielsen, C. Molecular Biopharmaceutics: Aspects of Drug Characterisation, Drug Delivery and Dosage Form Evaluation; Pharmaceutical Press: London, UK, 2010. [Google Scholar]
Sample Availability: Samples of compounds are available from authors. |






| Comp. |  | |||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | log P | log Sw | MV [cm3] | Parachor [cm3] | ST [dyne/cm] | |
| 1 | –(CH2)4– | −CH3 | −0.15 | −1.09 | 156.64 | 392.52 | 45.88 | |
| 2 | −H | −H | −H | −1.94 | 0.10 | 91.5 | 227.40 | 38.12 |
| 3 | −H | −H | −CH3 | −1.53 | −0.23 | 109.04 | 265.43 | 32.10 |
| 4 | −CH3 | −H | −CH3 | −1.15 | −0.55 | 126.22 | 303.45 | 28.36 |
| 5 | −CH3 | −CH3 | −CH3 | −0.79 | −0.75 | 142.53 | 341.84 | 27.03 |
| Time [h] | Control | Compounds | ||||
|---|---|---|---|---|---|---|
| 1 (alaptide) | 2 | 3 | 4 | 5 | ||
| 0.5 | 0.00 ± 0.00 | 0.79 ± 0.58 | 0.40 ± 0.24 | 0.39 ± 0.13 | 0.93 ± 0.18 | 0.32 ± 0.17 |
| 1 | 0.53 ± 0.12 | 7.92 ± 2.18 | 4.70 ± 1.17 | 2.52 ± 1.37 | 6.29 ± 1.01 | 2.44 ± 1.69 |
| 1.5 | 1.89 ± 0.33 | 20.53 ± 4.88 | 12.92 ± 2.08 | 7.54 ± 3.43 | 17.29 ± 2.37 | 8.22 ± 4.42 |
| 2 | 4.62 ± 0.60 | 33.66 ± 7.04 | 23.76 ± 3.04 | 14.77 ± 6.20 | 30.83 ± 3.59 | 16.16 ± 7.21 |
| 3 | 13.36 ± 1.63 | 68.73 ± 12.20 | 52.62 ± 5.18 | 34.41 ± 11.47 | 67.37 ± 6.53 | 39.36 ± 14.07 |
| 4 | 26.07 ± 3.31 | 114.08 ± 17.92 | 86.13 ± 7.16 | 59.64 ± 17.67 | 110.57 ± 9.03 | 69.44 ± 21.40 |
| 6 | 62.00 ± 8.40 | 212.49 ± 3062 | 168.24 ± 11.85 | 125.63 ± 28.83 | 216.87 ± 14.89 | 151.18 ± 37.41 |
| 8 | 112.06 ± 14.78 | 341.30 ± 45.25 | 270.85 ± 16.94 | 191.59 ± 37.56 | 319.76 ± 17.95 | 234.74 ± 49.44 |
| 12 | 235.20 ± 34.88 | 616.34 ± 72.86 | 496.43 ± 32.00 | 368.53 ± 56.04 | 595.79 ± 31.81 | 460.21 ± 77.54 |
| 24 | 684.56 ± 87.05 | 1439.30 ± 127.94 | 1253.20 ± 108.70 | 998.62 ± 120.08 | 1482.83 ± 74.42 | 1273.10 ± 144.46 |
| Comp. | J’1-3h [μg/cm2/h] | tlag’1-3h [h] | Kp’1-3h×10-3 [cm/h] | ERs’1-3h |
|---|---|---|---|---|
| control (theophylline) | 6.60 ± 0.80 | 1.10 ± 0.02 | 0.66 ± 0.08 | 1.00 ± 0.05 |
| 1 | 29.84 ± 7.02 | 0.77 ± 0.14 | 2.98 ± 0.70 | 4.52 ± 1.06 |
| 2 | 24.33 ± 2.05 | 0.91 ± 0.04 | 2.43 ± 0.20 | 3.68 ± 0.31 |
| 3 | 16.55 ± 3.92 | 0.87 ± 0.13 | 1.66 ± 0.39 | 2.51 ± 0.59 |
| 4 | 28.70 ± 1.73 | 0.80 ± 0.12 | 2.87 ± 0.17 | 4.35 ± 0.26 |
| 5 | 17.49 ± 5.48 | 0.91 ± 0.16 | 1.75 ± 0.55 | 2.65 ± 0.83 |
| Comp. | Jss [μg/cm2/h] | tlag [h] | Kp × 10−3 [cm/h] | ERs |
|---|---|---|---|---|
| control (theophylline) | 29.12 ± 3.93 | 3.74 ± 0.41 | 2.91 ± 0.39 | 1.00 ± 0.05 |
| 1 | 67.52 ± 9.19 | 2.91 ± 0.17 | 6.75 ± 0.92 | 2.32 ± 0.32 |
| 2 | 54.94 ± 3.46 | 2.99 ± 0.06 | 5.49 ± 0.35 | 1.89 ± 0.12 |
| 3 | 41.02 ± 4.77 | 3.13 ± 0.41 | 4.10 ± 0.48 | 1.41 ± 0.16 |
| 4 | 63.99 ± 3.25 | 2.77 ± 0.16 | 6.40 ± 0.33 | 2.20 ± 0.11 |
| 5 | 52.20 ± 6.74 | 3.31 ± 0.33 | 5.22 ± 0.67 | 1.79 ± 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokorna, A.; Bobal, P.; Oravec, M.; Rarova, L.; Bobalova, J.; Jampilek, J. Investigation of Permeation of Theophylline through Skin Using Selected Piperazine-2,5-Diones. Molecules 2019, 24, 566. https://doi.org/10.3390/molecules24030566
Pokorna A, Bobal P, Oravec M, Rarova L, Bobalova J, Jampilek J. Investigation of Permeation of Theophylline through Skin Using Selected Piperazine-2,5-Diones. Molecules. 2019; 24(3):566. https://doi.org/10.3390/molecules24030566
Chicago/Turabian StylePokorna, Aneta, Pavel Bobal, Michal Oravec, Lucie Rarova, Janette Bobalova, and Josef Jampilek. 2019. "Investigation of Permeation of Theophylline through Skin Using Selected Piperazine-2,5-Diones" Molecules 24, no. 3: 566. https://doi.org/10.3390/molecules24030566