Ecological Factors Determining Barking Deer Distribution and Habitat Use in the Mid-Hills of Nepal
- 1Institute of Forestry, Tribhuvan University, Pokhara, Nepal
- 2Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland
- 3Institute of Forestry, Tribhuvan University, Hetauda, Nepal
- 4Department of Biodiversity Research, Global Change Research Institute, Czech Academy of Sciences, Brno, Czechia
- 5Centre for Biology, Geoscience and Environmental Education, Faculty of Education, University of West Bohemia, Pilsen, Czechia
- 6Institute of Environmental Studies, Charles University, Prague, Czechia
Barking deer is found in dense tropical and subtropical forests of Asia. It is listed as “least concerned” by the International Union of Conservation of Nature and as “vulnerable” in Nepal, where it is also protected. Due to the habitat loss and fragmentation by human activities, barking deer abundance is decreasing, which may even ultimately lead to its extinction. This in turn might negatively affect local ecosystem such as the abundance of the endangered common leopard, for which barking deer is the main prey species in the mid-hills of Nepal. We therefore need to know factors affecting barking deer abundance and its habitat preferences. To determine these factors, we recorded barking deer either by direct sighting or by any evidence of its indirect presence observed through transect surveys in January and February, 2019. To analyze habitat preference, the presence of barking deer was set to 1 if the barking deer or any sign of its presence were observed “used plots,” or to 0 if the barking deer or any sign of its presence were not observed (“habitat availability plot”). We measured main four ecological drivers such as forest management regime, microclimate, disturbance and food resources, which include 11 habitat characteristics in spots where barking deer was present, and in randomly selected spots. We found that elevation, slope, distance from settlement, presence of tree species, depth of leaf litter and percentage cover of leaf litter were most significantly affecting its presence. These results can serve as guidelines for local authorities to prevent decline in abundance of barking deer.
Introduction
Mountain ungulates are an essential part of the Himalayan fauna (Schaller, 1977; Shackleton, 1997; Bagchi and Ritchie, 2010). Most of them are not endangered (IUCN, 2021) and therefore they are not in focus of conservation efforts. However, they serve as prey to large mammalian carnivores, which are endangered (Carbone and Gittleman, 2002; Karanth et al., 2004). Thus, decline of the abundances of mountain ungulates may negatively affect the abundances of carnivores, or even cause their extinction (Carbone and Gittleman, 2002; Karanth et al., 2004). In lack of their natural food, the carnivores then resort to hunting for food in human settlements, where they are killing mainly goats, but sometimes even people (Nyhus, 2016). The retaliation of the local villagers by killing the predators (Nowell et al., 2016) makes the situation even worse.
Hence, it is important to maintain large densities of mountain ungulates in order to prevent extinction of the large carnivores (Carbone and Gittleman, 2002; Karanth et al., 2004). Specifically, it is necessary to learn which are preferred habitats of the mountain ungulates and preserve them. It is also important to know which management regimes support the existence of suitable habitats for mountain ungulates.
The barking deer (Muntiacus vaginalis), also known as Northern Red Muntjac, is a nice example of this situation. It is a solitary mammal species found in dense tropical and subtropical forests of Asia (Oli and Jacobson, 1995; Liwei et al., 2004), listed as “least concerned” in Red Data Book of the International Union of Conservation of Nature (Timmins et al., 2016) and as “vulnerable” in Nepal (Jnawali et al., 2011). It is also protected under the National Parks and Wildlife Conservation Act 1973 of Nepal (Department of National Parks and Wildlife Conservation [DNPWC], 2017). Barking deer lives in elevations of 150–3,000 m, from lowland Terai (Gurung, 1993) to mid-hills of Nepal (Shrestha, 2005; Shrestha and Basnet, 2005; Nagarkoti and Thapa, 2007; Pokharel and Chalise, 2010) – see Figure 1.
Figure 1. Physiographic zones of Nepal. Source: S. Lamichhane, SSD, NARC; Data from Department of Survey 1996.
In low elevations (Terai), barking deer is a part of a rich community of various ungulates and therefore its density is not so important for the existence of large carnivores such as tiger (Panthera tigris) and common leopard (Panthera pardus). However, in higher elevations (mid-hills of Nepal), barking deer becomes a dominant deer species and therefore critical for survival of common leopard, the main predator there (Anup, 2017; Kandel, 2019). Barking deer constitutes a major part of common leopard’s diet in the mid-hills (Aryal and Kreigenhofer, 2009; Koirala et al., 2012; Shrestha, 2015; Kandel, 2019).
To ensure continuing abundance of the barking deer to secure relevant food source for local endangered large carnivores, we need to learn what are the preferred habitats for barking deer, maintain them, and – if possible – increase the habitats’ size. Such knowledge will help local authorities effectively manage the conservation of the barking deer and minimize any unwanted results of bad management, as for example the human-leopard conflicts.
Seen from a global perspective, a good understanding of factors determining the distribution of barking deer in the landscape provides important input data for modeling the dynamics of large mammal communities in the Nepalese midhills, of which barking deer is an important component. Population dynamics of these communities then determines the likelihood of survival of endangered species like common leopard.
Some studies on habitat preference (Jaenike and Holt, 1991) of barking deer were conducted outside of Nepal (Ohtaishi and Gao, 1990; Oka, 1998; Liwei et al., 2004; Hameed et al., 2009), but only a few we conducted directly in Nepal (Nagarkoti and Thapa, 2007; Pokharel et al., 2015; Lamichhane et al., 2020). Most of these papers studied only habitat use of barking deer but not factors affecting its distribution, including importance of ecological factors in each study such as microclimatic (e.g., elevation, slopes, aspect, and leaf litter), disturbances (e.g., proximity to settlements), food resources (e.g., trees and grass), and forest management regimes (see below). A detailed study of factors affecting habitat use and distribution of barking deer in Nepal is still lacking.
The dominant habitat of barking deer – forests – covers about 44.74% of the area of Nepal (Department of Forest Research and Survey [DFRS], 2015). The Forest Act of 2016 (second amendment) classifies forests as National and Private Forests (GoN, 2019). The National Forests are further sub-categorized as Government Managed Forests, Protected Forests (PFs), Community Forests (CFs), Collaborative Forests, Block Forests, Leasehold Forests, and Religious Forests (GoN, 2019). The CF belongs to local community, which performs its management for the joint benefits of both, the forest and local people (Pathak et al., 2017). However, only CFs were not sufficient for conservation of boundlessly growing populations of widespread and highly mobile species like barking deer. Therefore, particular forest patches were later declared as PFs to enhance the protection of biodiversity by the Government of Nepal (Shrestha et al., 2014). To generate and uphold a balance between conservation and human requirements for forest resources, Protected Forests Management Plan (PFMP) was developed, which assigns particular forest patches to particular functions (Shrestha et al., 2014). PFMP, in contrast with the community forestry regime, focuses basically on elevation of conservation benefits by including wildlife management into it while considering requirements of local communities (Shrestha et al., 2014). We have chosen these two forest management regimes (community and protected forests) to discover the effect of human intervention in the sustenance of barking deer and to find out its habitat preferences.
Materials and Methods
Study Area
The study was conducted in the mid-hills of Nepal (Figure 2), in two differentially managed forests:
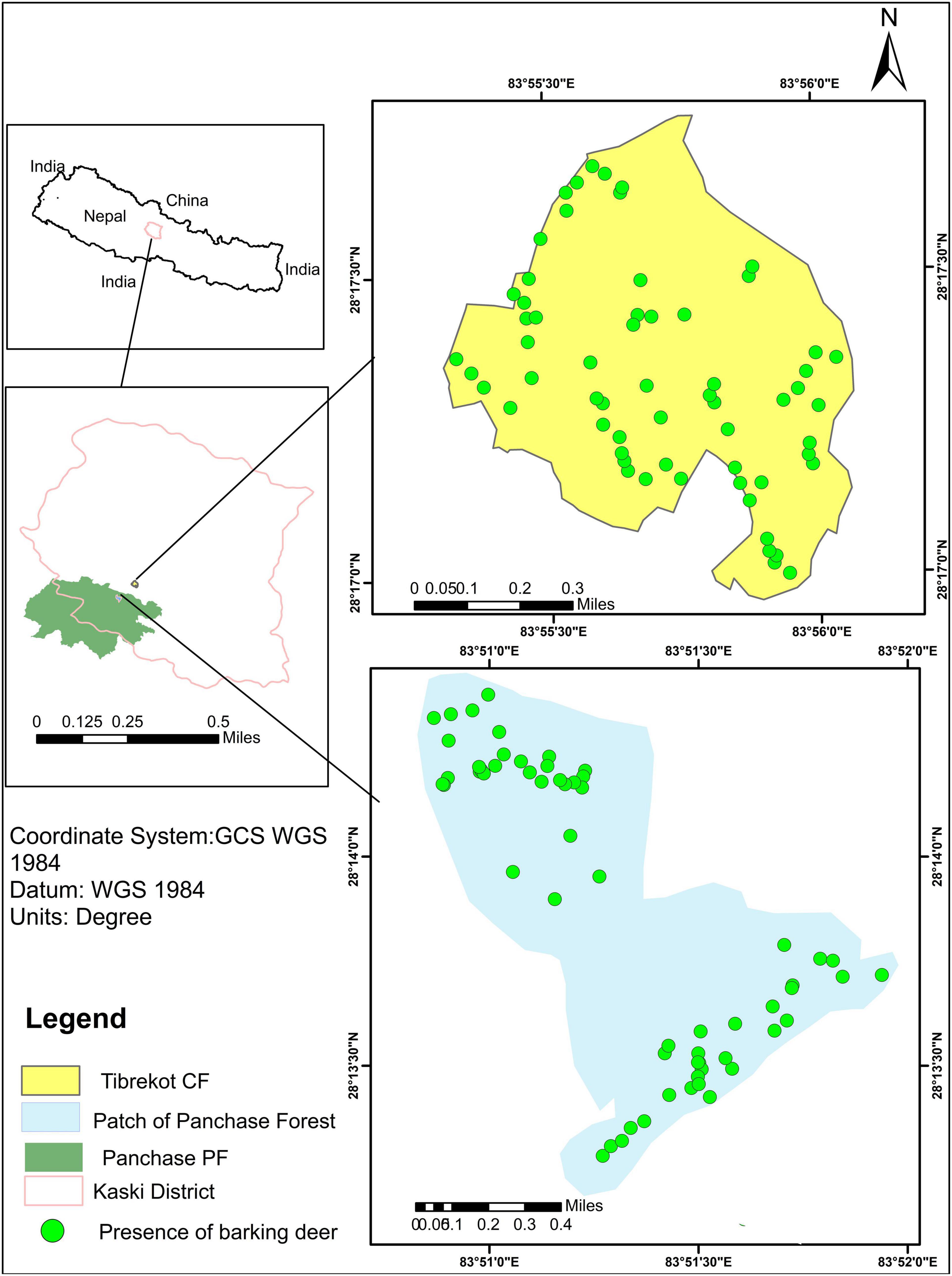
Figure 2. Map showing the location of study areas in Kaski Nepal: Tibrekot Community Forest (CF) and patch of Panchase Protected Forest (PF).
1. The Tibrekot CF (28.29oN, 83.93oE, 119.75 ha), in Hemja, Kaski, Nepal, which was handed over to the local community in 2000. Our study site covered its whole area.
2. The Panchase PF (28.2oN, 83.95oE, 5775.73 ha), situated at the junction of three districts: Kaski, Parbat and Syangja; the study area covered 130 ha within the PF.
The average elevation of both study sites was 1,000 m a.s.l. and their ecological and topographical features were similar. The dominant tree species in both sites are Castanopsis indica, Schima wallichii, Alnus nepalensis, Engelhardia spicata, and Myrica esculenta. P. pardus, Macaca mulatta, Canis aureus, and M. vaginalis are the main mammal species and Lophura leucomelanos, Zenaida auriculata, Corvus macrorhynchos, and Purpureicephalus spurius are the main bird species in these study sites.
Field Data Collection
For recording of barking deer we used a transect survey – a method widely used to monitor large mammals (Pokharel et al., 2015; Kunwar et al., 2016). The lengths of transects were 0.5–1.4 km at elevations of 900–1,400 m a.s.l. The total length of transects was 6.85 km in PF and 6.12 km in CF.
The transects were placed along walking trails (human trails used to collect fodder) and existing paths (minor trails used by domestic or wild animals), which have frequently been used to determine habitat use of wild ungulates (Sathyakumar, 1994; Ahmad et al., 2016; Kunwar et al., 2016; Syed and Ilyas, 2016). A possible bias appearing due to this transect design can be caused by roads that do not constitute a representative sample of habitats (Garton et al., 2004), or – for ungulates living in groups – there may be a strong negative relationship with human activities on trails open to all visitors (Blake et al., 2017). In our case, however, the transects were carefully chosen to represent all habitats, and barking deer is a solitary animal. We have used curvilinear transects to overcome the difficulties of working in steep, rugged and inaccessible terrain, as is often the case in the Himalaya (Sathyakumar, 1994).
We recorded all individuals we saw within 50 m distance at either side of a transect and any evidence of its indirect presence (pellets, hairs, and footprints) observed within 5 m distance at either side of the transect, following the method used by Pokharel and Chalise (2010). Two wildlife experts, two local people acquainted with the forests and two forest guards helped us to locate the direct sightings and indirect signs of barking deer. We recorded GPS coordinates of all barking deer individuals spotted (direct sightings) and indirect signs to prepare the distribution map using Arc GIS 10.8 version. The data was collected during January and February 2019, as then the signs of barking deer are more visible and there is a thinner vegetation in the forest (Skovlin, 1982; Parker et al., 1984; Wilson et al., 1996; Safford, 2004; Sanusi et al., 2013). We did this for 15 days in each study area, from 6:30–10:00 a.m. and from 4:30–6:00 p.m. – during the highest activities of the barking deer and other ungulates (Wilson et al., 1996; Pokharel and Chalise, 2010; Sanusi et al., 2013).
Indirect signs, such as pellets, cannot be easily recorded during the summer season because the forest ground cover is dense in summer and the rainfall easily washes away the pellets in the study area (high rainfall area of Nepal). Also, winter is the most difficult season for ungulates because of limited availability of food and water sources, which are crucial for their survival (Skovlin, 1982; Parker et al., 1984; Safford, 2004). Therefore, it is essential to study winter habitat use by barking deer.
The indirect signs of barking deer and domestic ungulates were differentiated as follows:
Domestic goats were mainly grazing near settlements and therefore there was less chance of confusion their indirect signs with those of barking deer, because the corresponding habitats were only weakly overlapping. In addition, based on literature (Gurung and Singh, 1996; Shrestha and Basnet, 2005), we identified the indirect signs of barking deer and domestic goats as follows:
1. Barking deer pellets are cylindrical in shape and often have a small point at one end. They are mostly found in clumps and adhere to each other. Domestic goat pellets are of elongated oval shape, smooth rounded at the ends and rarely stick together.
2. Barking deer hoof prints are pointed at the front and dew claw marks are visible in soft ground. Domestic goat hoof prints are rounded at the front and dew claw marks are never present.
Whenever any (direct or indirect) sign of barking deer was found, a circle with a 5 m radius and the center of the point where an animal or a sign of its presence were spotted was set up, following the method of Yahnke (2006) and Bernard et al. (2014). For each of these circles, one additional circle with a 5 m radius was set with the center localized 100 m apart from the former circle in a randomly chosen direction, according to Neupane et al. (2021). These plots represented average samples of habitat, independent of the presence/absence of the barking deer. In each of these circles, the presence of barking deer was set to 1 if the barking deer or any sign of its presence were observed in this circle (“used plots”), or to 0 if not (“habitat availability plot”). In all circles of both types, we measured 11 habitat characteristics (see Table 1 for their list). In total, we have used 134 “use plots” and 70 “habitat availability plots.” We did not distinguish between direct and indirect signs in the analyses.
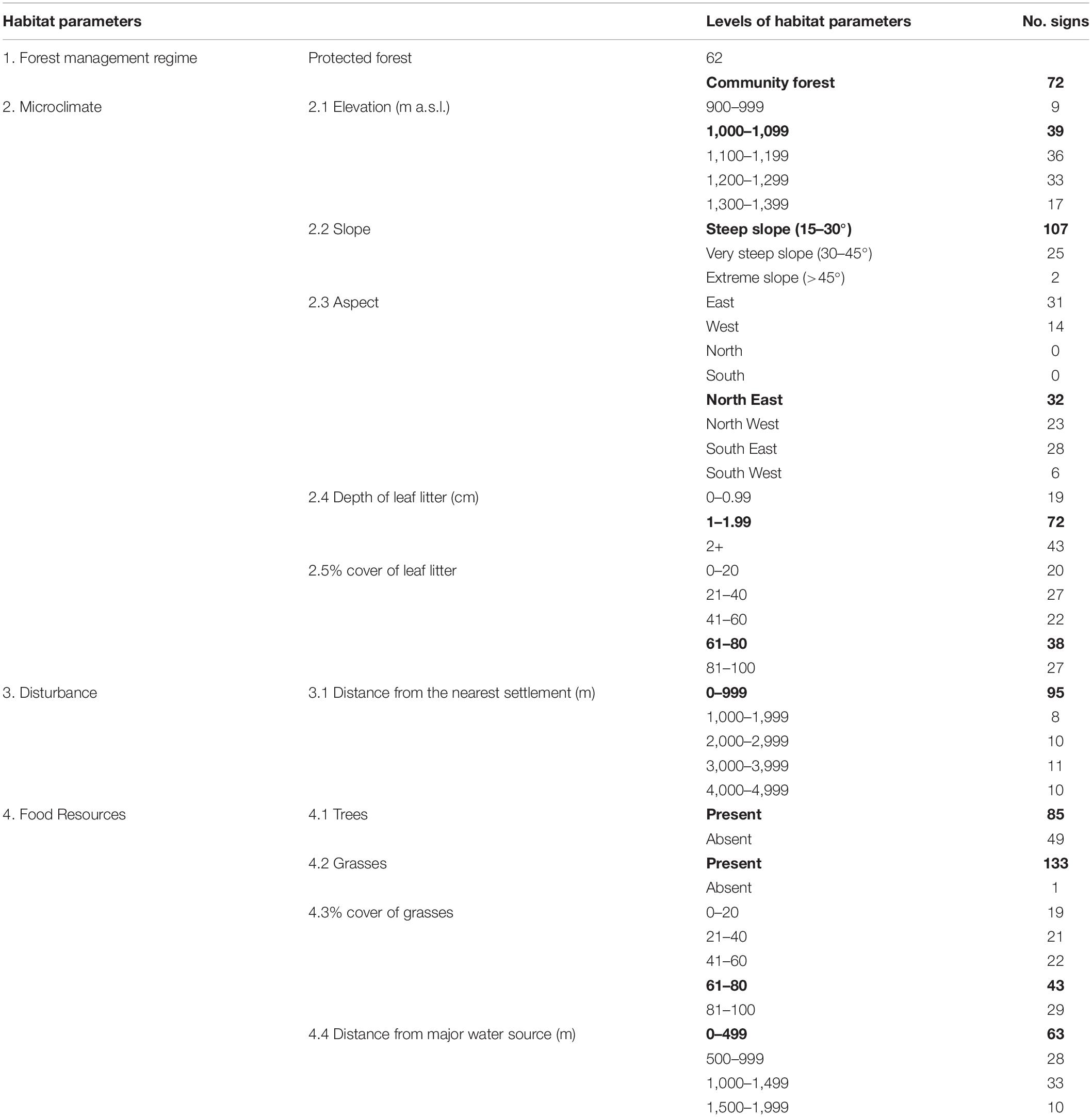
Table 1. Numbers of direct and indirect signs of barking deer presence for the 11 habitat parameters measured; data from both study areas are lumped; for each habitat parameter the most often recorded parameter levels are in boldface.
Data Analysis
To find out, which of the 11 habitat parameters selected (see Table 1 for the list) significantly affected the probability of presence of barking deer, generalized linear models (GLMs) with binomial distribution/logistic regression log(y/1−y) were used. Here, habitat parameters served as independent variables and presence of barking deer (depending on the type of circle: 1 – “use plot,” 0 – “habitat availability plot”) as dependent variable. Prior to the analyses, the parameters were tested for collinearity. Data from both sites (CF and PF) were lumped for these analyses. We have applied the backward selection method with target significance level 5% (significant χ2 likelihood ratio, p < 0.05). The habitat parameters that remained in the final model were considered as most significantly affecting the presence of the barking deer. We used “R × 64 4.0.3’’1 (R Core Team, 2020) for the calculations.
To get a more detailed view of the habitat parameter’s effects on the presence of barking deer, we have selected four habitat parameters as response variables, for which numerical (not categorial) data were available. We considered the parameters important based on the GLM modeling above, literature search, and our own field experience of what is important for barking deer. These response variables were: “distance from major water source,” “distance from the nearest settlement,” “percentage cover of grasses,” and “percentage cover of leaf litter.” For each of these variables, we have performed a 2-way factorial ANOVAs with factors “barking deer presence” and “site” (thus in these analyses the sites – CF and PF – were distinguished), and calculated means and standard errors for each combination of these response variables and each of the factors.
Results
Table 1 shows the numbers of both – used and habitat availability plots, where both direct and indirect signs of barking deer presence were recorded when data from both sites (CF or PF) were lumped. They are associated with individual habitat parameters. Table 2 shows those of the 11 habitat characteristics listed in Table 1, which had the strongest and statistically significant effect on the probability of presence of barking deer, according to the GLM models.
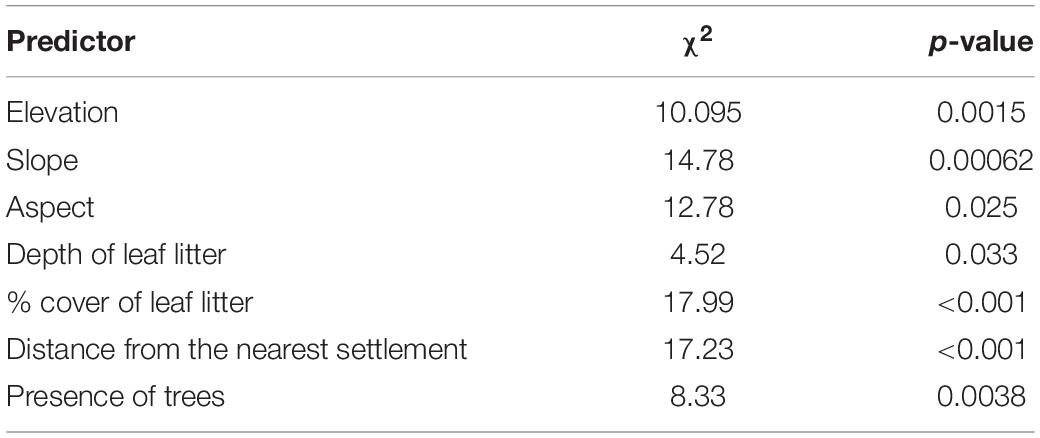
Table 2. Estimates and statistics for the final model predicting the probability of observing barking deer, according to the GLM models.
Results of the 2-way ANOVAs with factors “barking deer presence” and “site” for the four response variables selected are in Table 3. In most cases, the levels of factors significantly affected the response variable, except of “site” for “distance from nearest settlement,” “barking deer presence” for “percentage cover of grasses” and interactions “presence x site” for “distance from major water source” and “percentage cover of grasses” (Table 3).
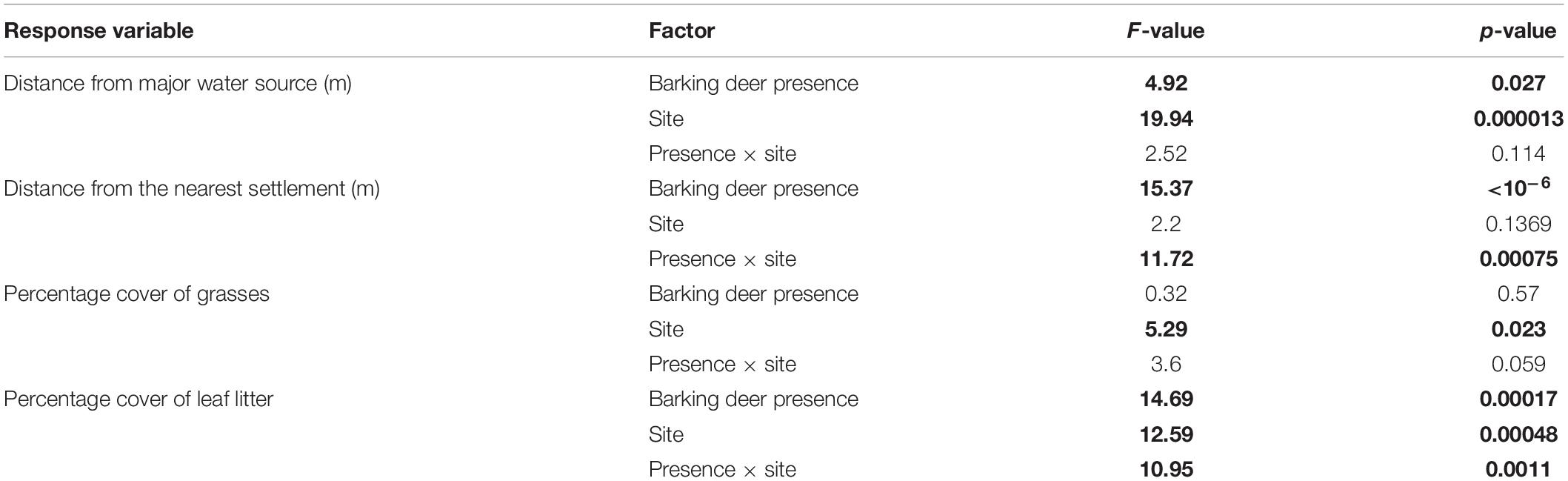
Table 3. Results of the 2-way factorial ANOVAs with factors “barking deer presence” and “site” for the response variables distance from major water source, distance from the nearest settlement, percentage cover of grasses and percentage cover of leaf litter; significant values (p < 0.05) are in boldface.
Figure 3 shows the means and standard errors for each combination of the response variables “distance from major water source,” “distance from the nearest settlement,” “percentage cover of grasses,” and “percentage cover of leaf litter” and each of the factors “barking deer presence” and “site.”
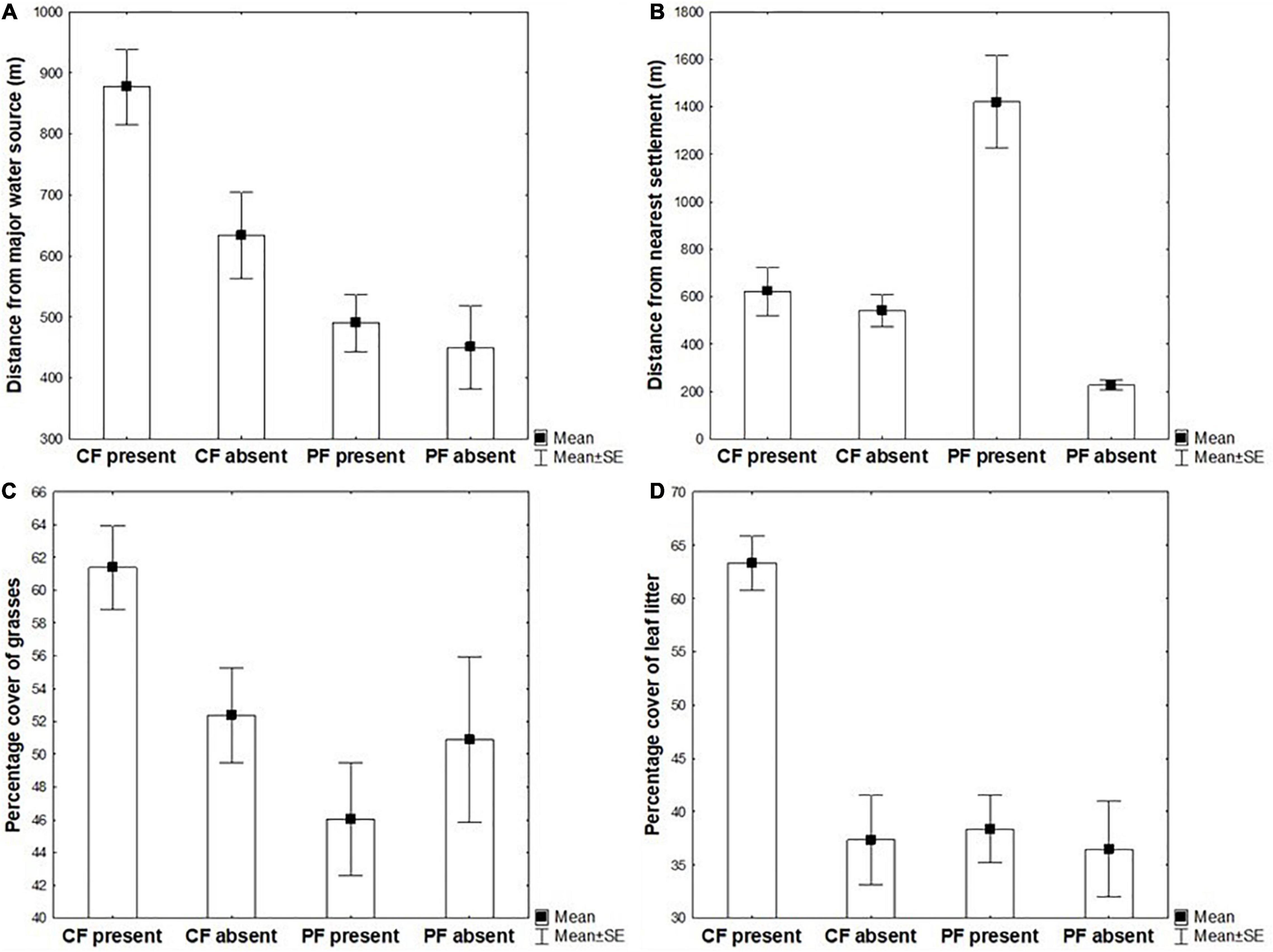
Figure 3. The means and standard errors for each combination of the response variables “distance from major water source” (A), “distance from the nearest settlement” (B), “percentage cover of grasse,” (C) and “percentage cover of leaf litter” (D) and each of the factors “barking deer presence” (present – “used plot,” absent – “habitat availability plot”) and “site” (Community Forest – CF, Protected Forest – PF).
There were less opportunities to find a water source in the CF as the average distance from major water source there was larger than that in the PF (Figure 3A). The differences were statistically significant (Table 3). In both types of forest, the distance from major water source was consistently larger in “used plots” – where signs of barking deer were present, compared with those where they were absent; this difference was not statistically significant in PF (Figure 3A and Table 3).
The distance from the nearest settlement was consistently larger in “used plots,” compared to the “habitat availability plots.” This was especially conspicuous and statistically significant in the PF but not statistically significant in the CF (Figure 3B and Table 3). This would indicate that barking deer is avoiding proximity of human settlements, especially when living in PFs.
The situation regarding percentage cover of grasses is unclear and no clear trends are visible (Figure 3C). Barking deer signs are usually found where the cover of grass is large in the CF, but the opposite is true in the PF. Probably some other factor may play a role here.
Regarding the percentage cover of leaf litter (Figure 3D), it is very conspicuous that the leaf litter is much more abundant where barking deer signs are present in the CF – this makes the interactions “presence x site” statistically significant. For the remaining three combinations of the two factors, the means were very similar and not statistically significantly different from each other.
Discussion
This is the first study on habitat preference of barking deer under two forest management regimes in the mid-hills of Nepal. Slightly more barking deer signs were observed in the CF compared to the PF, but the difference is statistically not significant. Thus we can only hypothesize that maybe a higher human disturbance in the PF might play a role here (Chikanbanjar et al., 2020). Another reason may be a higher availability of food, shelter and water sources in CF (pers. obs.).
Microclimate Factors
We found most barking deer signs in elevations 1,000–1,099 m a.s.l., but similar numbers were recorded in 1,100–1,199 m a.s.l. and in 1,200–1,299 m a.s.l. (Table 1). This indicates a large range of elevations, where barking deer can be found. Similarly, Timmins et al. (2016) claim that barking deer is widely distributed from the lowlands to the high mountains, i.e., that elevation alone does not directly affect the barking deer’s distribution. Instead, it is accepted that elevation is correlated with other climatic predictors such as precipitation, temperature and solar radiations (Elith and Leathwick, 2009) that lead to the change in habitat features and its quality to support the occurrence of the species.
We found that barking deer prefers north-east aspect (Table 1), which contradicts Hameed et al. (2009), who advocate for the southern aspect. Discussion here is difficult and mostly hypothetical: the north-east facing slopes may receive sunlight from early morning compared to the southern facing slopes (Nie et al., 1992), but exact data supporting either are missing.
Cover of leaf litter can also be linked with the forest cover, and the ungulates often choose the areas with forest cover when bedding material is required (Gill, 1966; Armstrong et al., 1983). In addition, deer is more selective in terms of thermal factors when selecting bedding sites (Staines, 1976; Wood, 1988; Mysterud and Østbye, 1995) and this could be the reason for choosing an area with proper litter coverage and suitable litter depth along with canopy cover, as this study indicates. Moen (1973) also concluded that bedded deer may lose the most significant amount of heat through conduction, i.e., through direct transfer between deer and substrate. This might be one of the reasons that the presence of barking deer was positively associated with depth and coverage of leaf litter. However, during day time, elk (Merrill, 1991) used humid substrates to increase heat loss through conduction to relieve heat stress.
Litter depth may decrease in the future: Hobbs (1996) predicts that ungulates can reduce litter thickness by removing litter, or by compacting litter through trampling. Thus, a similar study after a certain time would be good to evaluate the relationship of presence of deer (ungulates) and litter depth after some time. Ungulates can also increase the litter depth by selective feeding (Husheer et al., 2005) on broad-leaved plant species, replacing species composition toward conifers, which have more recalcitrant leaves, resulting in a stockpiling of litter over time. Deer is also suspected to influence edaphic characteristics by increasing soil temperature and salinity through exposure of bare soil after vegetation elimination through browsing, or by intensifying soil compaction through trampling which affects oxygen and soil water content (Schrama et al., 2013). Such alteration of the soil may also alter the decomposer capacity to degrade litter. Thus, a detailed study on plant litter dynamics due to presence of herbivores is needed.
Our results in Table 1 show that barking deer prefers some reasonable depth (more than 1 cm) and larger percentage cover of leaf litter (60–80%) in the habitat. This may be due to the use of leaf litter as a thermal insulator for bedding or as food. When facing food paucity, deer feed on dead leaves, bark and even underground parts of plants (Takahashi and Kaji, 2001). Figure 3D is less optimistic in this respect: in 3 out of 4 categories, just above 35% of leaf cover is sufficient for the barking deer. So again, probably some other factors may play a role here.
Predator avoidance. Barking deer is an important prey of tiger and common leopard (Wegge et al., 2009; Lovari et al., 2015). Most barking deer signs were observed at steep, but not very steep slopes (Pokharel and Chalise, 2010), in accord with our results (Table 2). Pokharel et al. (2015) suggest that this might be because predators and people may push the barking deer there as it is trying to avoid the risk of death (see also Ohtaishi and Gao, 1990; Paudel and Kindlmann, 2012).
Seasonal Variation in Distribution and Habitat Use
Previous studies indicate there is no significant difference in seasonal vegetation cover and no seasonal shift in elevation (Habiba et al., 2021) and no seasonal variation in home range size (Odden and Wegge, 2007). Therefore, our study in winter (January–February) would not cause a bias by using only a single season. Also in our experience, no seasonal shifts in elevation were observed in the mid-hills of Nepal.
Disturbance Factors
Barking deer is a selective browser, which feeds on flowers, twigs, fruits, bamboo shorts, foliage, bark, herbs, sprouts, seeds, grasses, birds eggs, carrion (Hofmann and Stewart, 1972; Jarman, 1974; Hofmann, 1989), and small mammals, which they kill and eat using their canines and forelegs (Humas, 2004). All this can be easily available in human-dominated agricultural landscape. As a result, barking deer is believed to like to live near to human settlements (Oka, 1998; Paudel and Kindlmann, 2012). If anything, then our data may slightly suggest the opposite, as the mean distance of observed signs of barking deer from human settlements in PF was much larger than the mean distance of the remaining three categories in Figure 3B. Again, Table 1 suggests the opposite, but it is questionable how many locations are really distant (e.g., more than 1,000 m) from any human settlement.
Food Resources Factors
Our results (Tables 1, 2) suggest that presence of not only grass, but also trees positively affects presence of barking deer, consistently with Liwei et al. (2004). That tree canopy cover and presence of herbs can directly influence barking deer presence was also shown by Hameed et al. (2009). Nagarkoti and Thapa (2007) and Roberts (1977) suggest that this may be because of the abundance of food, shelter and water sources in the forested area. Most of the ungulates seek refuge in forested areas at night, particularly during the dry season to escape from storms and cold (Dexter, 1998). In addition, barking deer also consumes fallen leaves and fruit within the areas covered by tree species (Lekagul and McNeely, 1977).
It is believed that the barking deer prefers to live close to water resources, as it drinks water at least once a day, usually in the morning or mid-day (Rafinesque., 1968; Yonzon, 1978). Lamichhane et al. (2020) also found that barking deer used Shorea robusta forest with the availability of water sources, in accord with Pokharel et al. (2015). However, our data are problematic in this respect. When the data from both sites were lumped, then most of the signs were found relatively close to the water sources: 0–499 m (Table 1). On the contrary, according to Figure 3A, in both types of forest, the distance from major water source was consistently larger in “used plots” – where signs of barking deer were present, compared with those where they were absent (“habitat availability plots”). The daily regime of data collection (6:30–10:00 a.m. and 4:30–6:00 p.m.) may explain this, at least for direct observations, as we will further. We do not have experience with daily movements of barking deer, but our observations of another mountain ungulate, blue sheep, tell us that they spend the morning (when the observations were made) by feeding in high elevations and toward the midday they slowly move down to river to drink. Later afternoon (when the second part of observations was made) they slowly return upward to higher elevations. This may lead to the observed above-average distance of the “used plots” from the water sources in Figure 3, although barking deer generally moves not far away from the water sources (Table 1).
Conclusion
We evaluated habitat preference of barking deer in two forests, differing in management regimes: Community Managed Forest and Government-managed PF in the mid-hills of Nepal. There were slightly more signs of barking deer presence in the CF, but the difference was not significant.
Presence of trees and high coverage for both grasses and leaf litter were the most preferred habitats by barking deer. Elevation, slope, distance to settlement, depth of leaf litter, and percentage cover of leaf litter were best correlated with the presence of barking deer. Thus, conservation efforts should concentrate on the maintenance and increase of the areas characterized by these factors.
By determining the major factors governing the distribution of barking deer in the Nepalese midhills we are providing important input data for modeling the dynamics of large mammal communities in the Nepalese midhills, of which barking deer is an important component. This addresses fundamental questions related to dynamical change in natural and managed systems, at least for the large mammal communities in Nepal. Our study is therefore important for modeling of population dynamics and ecology of these communities and subsequently their proper conservation.
It is necessary to mention that there can be also other factors affecting barking deer presence, which we did not consider here. These include ecological interactions between barking deer, predators and other wild ungulates.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because we used a transect survey using indirect signs and direct sighting to record the presence of animals.
Author Contributions
BN, BD, SB, BMS, SR, and SS collected the data, performed the first set of analyses, and wrote the first draft of the manuscript. IT performed the ANOVA analyses. PK, SV, and BS wrote the final version and reviewed the manuscript. PK improved the concept of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Institute of Forestry Dean Office (as the Faculty Strategic Research Grant under NORHED funded IOF grant no: 585-075-076) supported by SUNREM-Himalaya, NORHED South driven project, https://www.iofpc.edu.np/project/norhed-project. BS, IT, and PK were supported by the Czechglobe institutional grant and by the Ministry of Education, Youth and Sports of CR within the CzeCOS program, grant number LM2018123.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to Mannshanta Ghimire of Pokhara Bird Society (PBS) and his team for assisting us during the barking deer sign identification and habitat survey. Similarly, we provide sincere gratitude to Krishna Raj Tiwari, Santosh Rayamajhi, Thakur Silwal, Nirjala Raut, Giri Raj Poudel, and the staffs of IOF for their contributions to accomplish this study. We appreciate the help of Laxman Kunwar, Shalikram Kandel, Samjhana Karki, Prativa Bhandari, Ambika Regmi, Manita Parajuli, Prabin Poudel, and Pawan Karki and of field experienced local people of Tibrekot Community Forest, Hemja, Kaski, and Panchase Protected Forest, Kaski for their generous support during the fieldwork.
Footnotes
References
Ahmad, K., Qureshi, Q., Agoramoorthy, G., and Nigam, P. (2016). Habitat use patterns and food habits of the Kashmir red deer or Hangul (Cervus elaphus hanglu) in Dachigam National Park. Kashmir, India. Ethol. Ecol. Evol. 28, 85–101. doi: 10.1080/03949370.2015.1018955
Anup, K. (2017). Community forestry management and its role in biodiversity conservation in Nepal. Glob. Expo. Wildl. Manag. 4, 51–72.
Armstrong, E., Euler, D., and Racey, G. (1983). Winter bed-site selection by white-tailed deer in central Ontario. J. Wildl. Manag. 47, 880–884. doi: 10.2307/3808632
Aryal, A., and Kreigenhofer, B. (2009). Summer diet composition of the common leopard Panthera pardus (Carnivora: Felidae) in Nepal. J. Threat. Taxa 1, 562–566. doi: 10.11609/JoTT.o2287.562-6
Bagchi, S., and Ritchie, M. E. (2010). Herbivore effects on above- and belowground plant production and soil nitrogen availability in the trans-Himalayan shrub-steppes. Oecology 164, 1075–1082. doi: 10.1007/s00442-010-1690-5
Bernard, H., Baking, E. L., Giordano, A. J., Wearn, O. R., and Ahmad, A. H. (2014). Terrestrial mammal species richness and composition in three small forest patches within an oil palm landscape in Sabah. Malaysian Borneo. Mamm. Study 39, 141–154. doi: 10.3106/041.039.0303
Blake, J. G., Mosquera, D., Loiselle, B. A., Romo, D., and Swing, K. (2017). Effects of human traffic on use of trails by mammals in lowland forest of eastern Ecuador. Neotrope. Biodivers. 3, 57–64. doi: 10.1080/23766808.2017.1292756
Carbone, C., and Gittleman, J. L. (2002). A common rule for the scaling of carnivore density. Science 295, 2273–2276. doi: 10.1126/science.1067994
Chikanbanjar, R., Baniya, B., and Dhamala, M. K. (2020). An Assessment of forest structure, regeneration status and the impact of human disturbance in Panchase Protected Forest. Nepal. Forestry 17, 42–66. doi: 10.3126/forestry.v17i0.33621
Department of Forest Research and Survey [DFRS] (2015). State of Nepal’s Forests. Forest Resource Assessment (FRA) Nepal. Kathmandu: Department of Forest Research and Survey (DFRS).
Department of National Parks and Wildlife Conservation [DNPWC] (2017). Profiling of protected and human wildlife conflicts associated wild animals in Nepal. Kathmandu: Department of National Parks and Wildlife Conservation.
Dexter, N. (1998). The influence of pasture distribution and temperature on habitat selection by feral pigs in a semi-arid environment. Wildl. Res. 25, 547–559. doi: 10.1071/WR97119
Elith, J., and Leathwick, J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697. doi: 10.1146/annurev.ecolsys.110308.120159
Garton, E. O., Ratti, J. T., and Giudice, J. H. (2004). “Research and experimental design,” in Techniques for Wildlife Investigations and Management, ed. C. E. Braun (Maryland, WA: The Wildlife Society).
GoN (2019). The Forests Act, 2019 (2076). Available online at: https://www.lawcommission.gov.np/en/wp-content/uploads/2021/03/The-Forest-Act-2019-2076.pdf (accessed on Mar 20, 2021).
Gurung, K. K., and Singh, R. (1996). Field Guide to the Mammals of the Indian subcontinent. Indian Experience. Oxford: Academic Press.
Gurung, M. K. (1993). An Assessment of the Habitat Model to Predict Distribution and Habitat Pattern of Large Mammals in the Annapurna Area, Ph.D thesis, Kirtipur: Tribhuvan University.
Habiba, U., Anwar, M., Khatoon, R., Hussain, M., Khan, K. A., Khalil, S., et al. (2021). Feeding habits and habitat use of barking deer (Muntiacus vaginalis) in Himalayan foothills. Pakistan. PLoS One 16:e0245279. doi: 10.1371/journal.pone.0245279
Hameed, W., and Fakhar-i-Abbas Mian, A. (2009). Population features of barking deer (Muntiacus muntjak) in Margalla Hills National Park. Pakistan. Pakistan J. Zool. 41, 137–142.
Hobbs, N. T. (1996). Modification of ecosystems by ungulates. J. Wildl. Manag. 60, 695–713. doi: 10.2307/3802368
Hofmann, R. R. (1989). Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecology 78, 443–457. doi: 10.1007/BF00378733
Hofmann, R. R., and Stewart, D. R. M. (1972). Grazer or browser: a classification based on the stomach-structure and feeding habits of East African ruminants. Mammalia 36, 226–240. doi: 10.1515/mamm.1972.36.2.226
Humas (2004). Kiang Muntiacus muntjac. Available online at: http://www.tamansafari.com/ (accessed on Mar 20, 2020).
Husheer, S. W., Hansen, Q. W., and Urlich, S. C. (2005). Effects of red deer on tree regeneration and growth in Aorangi Forest. Wairarapa. N. Z. J. Ecol. 29, 271–277.
IUCN (2021). The IUCN Red List of Threatened Species. Version 2021-2. Available online at: https://www.iucnredlist.org (accessed on Sep 19, 2021).
Jaenike, J., and Holt, R. D. (1991). Genetic variation for habitat preference: evidence and explanations. Am. Nat. 137, S67–S90. doi: 10.1038/nature07285
Jarman, P. (1974). The social organization of antelope in relation to their ecology. Behavior 48, 215–267.
Jnawali, S. R., Baral, H. S., Lee, S., Acharya, K. P., Upadhyay, G. P., Pandey, M., et al. (2011). The status of Nepal mammals: The National Red List Series. Kathmandu: Department of National Parks and Wildlife Conservation.
Kandel, S. R. (2019). Panthera pardus fusca (Family: Felidae) diet composition from Lamjung. Nepal. Environ. Ecol. Res. 7, 253–258.
Karanth, K. U., Nichols, J. D., Kumar, N. S., Link, W. A., and Hines, J. E. (2004). Tigers and their prey: predicting carnivore densities from prey abundance. Proc. Natl. Acad. Sci. U. S. A. 6, 4854–4858. doi: 10.1073/pnas.0306210101
Koirala, R. K., Aryal, A., Amiot, C., Adhikari, B., Karmacharya, D., and Raubenheimer, D. (2012). Genetic identification of carnivore scat: implication of dietary information for human–carnivore conflict in the Annapurna Conservation Area. Nepal. Zool. Ecol. 22, 137–143. doi: 10.1080/21658005.2012.744864
Kunwar, A., Gaire, R., Pokharel, K. P., Baral, S., and Thapa, T. B. (2016). Diet of the four-horned antelope Tetracerus quadricornis (De Blainville, 1816) in the Churia hills of Nepal. J. Threat. Taxa 8, 8745–8755. doi: 10.11609/jott.1818.8.5.8745-8755
Lamichhane, S., Khanal, G., Karki, J. B., Aryal, C., and Acharya, S. (2020). Natural and anthropogenic correlates of habitat use by wild ungulates in Shuklaphanta National Park. Nepal. Glob. Ecol. Conserv. 24:e01338. doi: 10.1016/j.gecco.2020.e01338
Lekagul, B., and McNeely, J. A. (1977). Mammals of Thailand. Kulusapa: Association for the conservation of Wildlife.
Liwei, T. E. N. G., Zhensheng, L. I. U., Yan-Ling, S. O. N. G., and Zhigao, Z. E. N. G. (2004). Forage and bed sites characteristics of Indian muntjac (Muntiacus muntjak) in Hainan Island. China. Ecol. Res. 19, 675–681. doi: 10.1111/j.1440-1703.2004.00683.x
Lovari, S., Pokheral, C. P., Jnawali, S. R., Fusani, L., and Ferretti, F. (2015). Coexistence of the tiger and the common leopard in a prey-rich area: the role of prey partitioning. J. Zool. 295, 122–131. doi: 10.1111/jzo.12192
Merrill, E. H. (1991). Thermal constraints on use of cover types and activity time of elk. Appl. Anim. Behav. Sci. 29, 251–267. doi: 10.1016/0168-1591(91)90252-S
Mysterud, A., and Østbye, E. (1995). Bed-site selection by European roe deer Capreolus capreolus in southern Norway during winter. Can. J. Zool. 73, 924–932. doi: 10.1139/z95-108
Nagarkoti, A., and Thapa, T. B. (2007). Distribution pattern and habitat preference of barking deer Muntiacus muntjac in Nagarjun forest. Kathmandu. Himalayan J. Sci. 4, 70–74. doi: 10.3126/hjs.v4i6.985
Neupane, B., Chhetri, N. B., and Dhami, B. (2021). Habitat selection of Himalayan Musk Deer Moschus leucogaster (Mammalia: Artiodactyla: Moschidae) with respect to biophysical attributes in Annapurna Conservation Area of Nepal. J. Threat. Taxa 13, 18703–18712.
Nie, D., Demetriades-Shah, T., and Kanemasu, A. E. (1992). Surface energy fluxes on four slope sites during FIFE 1988. J. Geophys. Res. Atmos. 97, 18641–18649. doi: 10.1029/91JD03043
Nowell, K., Li, J., Paltsyn, M., and Sharma, R. K. (2016). An Ounce of Prevention: Snow Leopard Crime Revisited. Cambridge, UK: TRAFFIC.
Nyhus, P. J. (2016). Human-wildlife conflict and coexistence. Annu. Rev. Environ. Resour. 41, 143–171. doi: 10.1146/annurev-environ-110615-085634
Odden, M., and Wegge, P. (2007). Predicting spacing behavior and mating systems of solitary cervids: a study of hog deer and Indian muntjac. Zoology 110, 261–270. doi: 10.1016/j.zool.2007.03.003
Ohtaishi, N., and Gao, Y. (1990). A review of the distribution of all species of deer (Tragulidae. Moschidae and Cervidae) in China. Mamm. Rev. 20, 125–144. doi: 10.1111/j.1365-2907.1990.tb00108.x
Oka, G. M. (1998). Factors Affecting the Management of Muntjac Deer Muntiacus Muntjak in Bali Barat National Park, Indonesia, Ph.D thesis, Australia: Western Sydney University.
Oli, M. K., and Jacobson, H. A. (1995). Vocalizations of barking deer Muntiacus muntjak in Nepal. Mammalia 59, 179–186. doi: 10.1515/mamm.1995.59.2.179
Parker, K. L., Robbins, C. T., and Hanley, T. A. (1984). Energy expenditures for locomotion by mule deer and elk. J. Wildl. Manag. 48, 474–487.
Pathak, B. R., Yi, X., and Bohara, R. (2017). Community based forestry in Nepal: Status, issues and lessons learned. Int. J. Sci. 6, 119–129.
Paudel, P. K., and Kindlmann, P. (2012). Human disturbance is a major determinant of wildlife distribution in Himalayan midhill landscapes of Nepal. Anim. Conserv. 15, 283–293. doi: 10.1111/j.1469-1795.2011.00514.x
Pokharel, K., and Chalise, M. K. (2010). Status and distribution pattern of barking deer Muntiacus muntjak in Hemja VDC. Kaski. Nepal J. Sci. Technol. 11, 223–228. doi: 10.3126/njst.v11i0.4149
Pokharel, K. P., Ludwig, T., and Storch, I. (2015). Spatial niche partitioning in sub-tropical solitary ungulates: four-horned antelope and barking deer in Nepal. PLoS One. 10:e0117917. doi: 10.1371/journal.pone.0117917
R Core Team (2020). R: A language and environment for statistical computing. Austria: R Foundation for Statistical Computing.
Rafinesque. (1968). Artiodactyla; Cervidae; Genus: Muntiacus. In: EP Walker, 1968, Mammals of the World (II edition). Baltimore: Johns Hopkins Press.
Safford, R. K. (2004). Modelling critical winter habitat of four ungulate species in the Robson Valley. British Columbia. J. Ecosyst. Manag. 4, 1–14.
Sanusi, M. A., Shukor, M. A., Wan Julian, W. A., and Traeholt, C. (2013). Activity Pattern of Selected Ungulates at Krau Wildlife Reserve. AIP Conf. Proc. 1571, 325–330. doi: 10.1063/1.4858677
Sathyakumar, S. (1994). Habitat ecology of major ungulates in Kedarnath Musk Deer Sanctuary, Western Himalaya, Ph.D thesis, Rajkot: Saurashtra University.
Schaller, G. B. (1977). Mountain Monarchs: Wild Sheep and Goats of the Himalaya. Chicago: University of Chicago Press.
Schrama, M., Heijning, P., Bakker, J. P., van Wijnen, H. J., Berg, M. P., and Olff, H. (2013). Herbivore trampling as an alternative pathway for explaining differences in nitrogen mineralization in moist grasslands. Oecology 172, 231–243. doi: 10.1007/s00442-012-2484-8
Shackleton, D. M. (1997). “Why Caprinae?,” in Wild Sheep and their Relatives: Status Survey and Conservation Action Plan for Caprinae, ed. D. M. Shackleton (Cambridge, UK: IUCN Gland), 5–7.
Shrestha, B. (2005). Distribution and Diversity of Mammals with Reference to Disturbance in Shivapuri National Park, Ph.D thesis, Kirtipur: Central Department of Zoology, Tribhuvan University.
Shrestha, B., and Basnet, K. (2005). Indirect method of identifying mammals: a case study from Shivapuri National Park. Nepal. Int. J. Ecol. 12, 43–58. doi: 10.3126/eco.v12i0.3196
Shrestha, P. M. (2015). Diet Composition of Leopard (Panthera pardus Linnaeus, 1758) in Shivapuri Nagarjun National Park, Nepal, Ph.D thesis, Kirtipur: Central Department of Zoology, Tribhuvan University.
Shrestha, T. K., Aryal, A., Rai, R. K., Lamsal, R. P., Koirala, S., Jnawali, D., et al. (2014). Balancing wildlife and human needs: the protected forest approach in Nepal. Nat. Areas J. 34, 376–380. doi: 10.3375/043.034.0313
Skovlin, J. M. (1982). “Habitat requirements and evaluations,” in Elk of North America: Ecology and Management, eds J. W. Thomas and D. E. Toweill (Harrisburg, Pa: Stackpole Books, Wildlife Management Institute), 369–413.
Staines, B. W. (1976). The use of natural shelter by red deer Cervus elaphus in relation to weather in North-east Scotland. J. Zool. 180, 1–8. doi: 10.1111/j.1469-7998.1976.tb04658.x
Syed, Z., and Ilyas, O. (2016). Habitat preference and feeding ecology of alpine musk deer (Moschus chrysogaster) in Kedarnath Wildlife Sanctuary, Uttarakhand, India. Anim. Prod. Sci. 56, 978–987. doi: 10.1071/AN141028
Takahashi, H., and Kaji, K. (2001). Fallen leaves and unpalatable plants as alternative foods for sika deer under food limitation. Ecol. Res. 16, 257–262. doi: 10.1046/j.1440-1703.2001.00391.x
Timmins, R. J., Steinmetz, R., Samba Kumar, N., Anwarul Islam, M. D., and Sagar Baral, H. (2016). Muntiacus vaginalis. The IUCN Red List of Threatened Species 2016: e.T136551A22165292. Available online at: http://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T136551A22165292.en (accessed on Sep 20, 2021).
Wegge, P., Odden, M., Pokharel, C. P., and Storaas, T. (2009). Predator-prey relationships and responses of ungulates and their predators to the establishment of protected areas: a case study of tigers, leopards and their prey in Bardia National Park. Nepal. Biol. Conserv. 142, 189–202. doi: 10.1016/j.biocon.2008.10.020
Wilson, D. E., Cole, F. R., Nichols, J. D., Rudran, R., and Foster, M. S. (1996). Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Washington, DC: Smithsonian Institution Press.
Yahnke, C. J. (2006). Habitat use and natural history of small mammals in the central Paraguayan Chaco. Mastozool. Neotrope. 13, 103–116.
Keywords: barking deer, habitat preference, mid-hills of Nepal, Muntiacus vaginalis, wild ungulate
Citation: Neupane B, Dhami B, Bista S, Sadadev BM, Regmi S, Shrestha S, Shrestha B, Traxmandlová I, Varachova S and Kindlmann P (2022) Ecological Factors Determining Barking Deer Distribution and Habitat Use in the Mid-Hills of Nepal. Front. Ecol. Evol. 10:894369. doi: 10.3389/fevo.2022.894369
Received: 11 March 2022; Accepted: 17 May 2022;
Published: 09 June 2022.
Edited by:
Pedro J. Leitão, Leipzig University, GermanyReviewed by:
Emilio Civantos, Centro de Investigacao em Biodiversidade e Recursos Geneticos (CIBIO-InBIO), PortugalMuhammad Kabir, The University of Haripur, Pakistan
Copyright © 2022 Neupane, Dhami, Bista, Sadadev, Regmi, Shrestha, Shrestha, Traxmandlová, Varachova and Kindlmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bikram Shrestha, bikramone@gmail.com
 Bijaya Neupane
Bijaya Neupane Bijaya Dhami
Bijaya Dhami Shreyashi Bista1
Shreyashi Bista1  Sami Shrestha
Sami Shrestha Bikram Shrestha
Bikram Shrestha Iva Traxmandlová
Iva Traxmandlová Sona Varachova
Sona Varachova Pavel Kindlmann
Pavel Kindlmann