The Evolution of Alternative Buoyancy Mechanisms in Freshwater Fish Eggs
- 1Donghu Experimental Station of Lake Ecosystems, State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Department of Ecology and Vertebrate Zoology, University of Łódź, Łódź, Poland
- 4Institute of Vertebrate Biology, Academy of Sciences of the Czech Republic, Brno, Czechia
- 5Institute for Ecological Research and Pollution Control of Plateau Lakes, School of Ecology and Environmental Science, Yunnan University, Kunming, China
Alternative reproductive tactics (ARTs) are behavioural, morphological, and physiological traits associated with alternative reproductive phenotypes within a population or species. ARTs are widespread in nature, and are a particular feature of teleost fishes. However, few studies have examined egg buoyancy mechanisms in the context of the evolution of ARTs in freshwater fishes. In marine fishes, egg buoyancy is achieved chiefly through hydration. While the buoyancy of freshwater fish eggs has been suggested to be determined primarily through the presence of oil droplets, the majority (60%) of freshwater pelagic eggs do not possess an oil droplet. We applied a physical model of buoyancy to understand the contributions of oil droplets and hydration to the buoyancy of pelagic freshwater fish eggs. We further used phylogenetic regression to estimate the effect of the relative size of the perivitelline space, habitat and parental care on the occurrence of oil droplets, while controlling for non-independence among species due to phylogenetic relatedness. Our analysis demonstrates that the probability of oil droplets in freshwater pelagic eggs exhibits a significant negative relationship with the size of perivitelline space, which may reflect a trade-off relating to energy allocation in contrasting habitats. We also demonstrate a positive association between the probability of oil droplets and the provision of parental care and occupancy of lentic habitats. These findings indicates the evolution of contrasting buoyancy mechanisms as novel ARTs in freshwater fishes. A theoretical model in combination with empirical analysis indicate the evolution of novel ARTs in freshwater fishes as adaptive responses to flow conditions.
Introduction
Understanding the processes by which alternative phenotypes evolve and are maintained within species, populations and taxa is a central objective in evolutionary biology (West-Eberhard, 1986; Smith and Skúlason, 1996; Oliveira et al., 2008; Taborsky and Brockmann, 2010). Alternative reproductive tactics (ARTs) are behavioural, morphological, and physiological traits associated with contrasting reproductive phenotypes within a population or species, and represent striking examples of population and taxonomic diversity (Stiver et al., 2015; Monroe et al., 2016). Despite extensive variation in ARTs in many taxonomic groups (Oliveira et al., 2008), the evolution of ARTs is incompletely understood. Since teleost fishes show the greatest variability of ARTs among vertebrates, they represent an outstanding model system for the study of the evolution of ARTs (Taborsky, 2008).
Teleost fishes express a reproductive tactic of high fecundity and small egg size (Wootton and Smith, 2015). Within this broad tactic, variation in the size, composition and distribution of fish eggs can play a critical role in egg and larval survival (Wootton, 1994; Kamler, 2005; Wootton and Smith, 2015). Some fishes produce pelagic eggs, while others produce demersal or adhesive eggs (Kunz-Ramsay, 2013; Wootton and Smith, 2015). Pelagic eggs appear to have evolved in response to several selective forces, including nutrient enrichment, nutrient concentration, propagule retention (Fundamental Triad Process), and avoidance of larval competition and predation (Loophole Process) (Hoagstrom and Turner, 2015). However, pelagic egg buoyancy mechanisms in the context of the evolution of ARTs in freshwater fishes are still unknown.
In the marine environment approximately 70% of teleost species produce pelagic eggs (Baras et al., 2018). The osmolality of marine eggs is lower than that of seawater, but higher than that of ovarian fluid (Finn et al., 2002; Wootton and Smith, 2015; Sørensen et al., 2016). Thus in seawater, buoyancy can be achieved through hydration of eggs in the ovary without the need to invest in energy-rich oil droplets. The evolution of effective egg hydration has been considered a major evolutionary innovation by teleost fishes that has allowed them to populate the open ocean (Wootton and Smith, 2015). While about 81% of marine fishes produce pelagic eggs with oil droplets, the average volume of these oil droplets is only 2% of total egg volume (Baras et al., 2018). In addition, many marine pelagic species lack oil droplets, while some species with demersal eggs have lipid contents equivalent to those of pelagic species. These observations suggest that in marine species egg buoyancy is primarily achieved through hydration rather than the presence of low-density oil droplets (Craik and Harvey, 1987; Thorsen et al., 1996; Fabra et al., 2005; Sundby and Kristiansen, 2015).
For freshwater fishes, the osmolality of eggs is higher than that of freshwater, which means that egg buoyancy is less readily achieved by hydration alone. In this case, the lipid content of freshwater eggs has been assumed to play the primary role in achieving neutral or positive buoyancy (Wootton and Smith, 2015; Baras et al., 2018). Among 351 freshwater fishes for which data were available, the proportion of species with pelagic eggs was estimated to be 21% (Baras et al., 2018), including many species of economic importance, such as grass carp (Ctenopharyngodon idella) and bighead carp (Hypophthalmichthys nobilis) (FAO, 2018). However, despite the commercial importance of several freshwater species with pelagic eggs, our understanding of egg buoyancy mechanisms in freshwater species is incomplete. In particular, an unresolved question is why only 40% of species with pelagic eggs possess oil droplets and, by extension, what egg buoyancy mechanisms operate in the eggs of the remaining 60% of freshwater species with pelagic eggs that do not contain oil droplets (Baras et al., 2018). Many freshwater species produce pelagic eggs that have a specific density only slightly greater than that of water. In these species the eggs become suspended in the water column when experiencing flow. By remaining in the water column, these pelagic eggs benefit from exposure to well-oxygenated water, while avoiding abrasive damage from coarse substrates (Mueller et al., 2013).
Here we explore the evolution of egg buoyancy mechanisms in freshwater fishes by extending the model of Sundby and Kristiansen (2015) to investigate the forces involved in egg buoyancy. We compiled data for the volume of oil droplets and perivitelline spaces of 55 of the 85 freshwater fishes with pelagic eggs for which data were available, and used a phylogenetically-corrected regression analysis to identify key variables associated with the presence of oil droplets. We predicted that the volume of oil droplets in an egg would correlate negatively with relative size of the perivitelline space, reflecting two alternative egg buoyancy mechanisms. Further, based on phylogenetic regression we estimate the effect of the occupancy of lentic habitats and provision of parental care on the occurrence of oil droplets, and discuss the evolution of ARTs in freshwater fish pelagic eggs in the context of condition dependence.
Materials and Methods
Data
Information on scientific names, habitat preference and expression of parental care were primarily obtained from FishBase,1 with additional sources identified where relevant. Of 85 freshwater fish species with pelagic eggs, data for pre- and post-hydrated egg diameter and size of oil droplets, if present, were compiled for 55 species from published studies (Supplementary Table 1). Because pelagic eggs are uniformly spherical, egg volume can be calculated from egg diameter as:
Where de is egg diameter. The total volume of oil droplets was estimated as the sum of individual oil droplet volumes (designated OD). The perivitelline space fills with water between the chorion and the embryo and yolk (Figure 1). The relative size of the perivitelline space (PS) volume was calculated as the difference in volume between pre- and post-hydrated eggs (Supplementary Table 1).
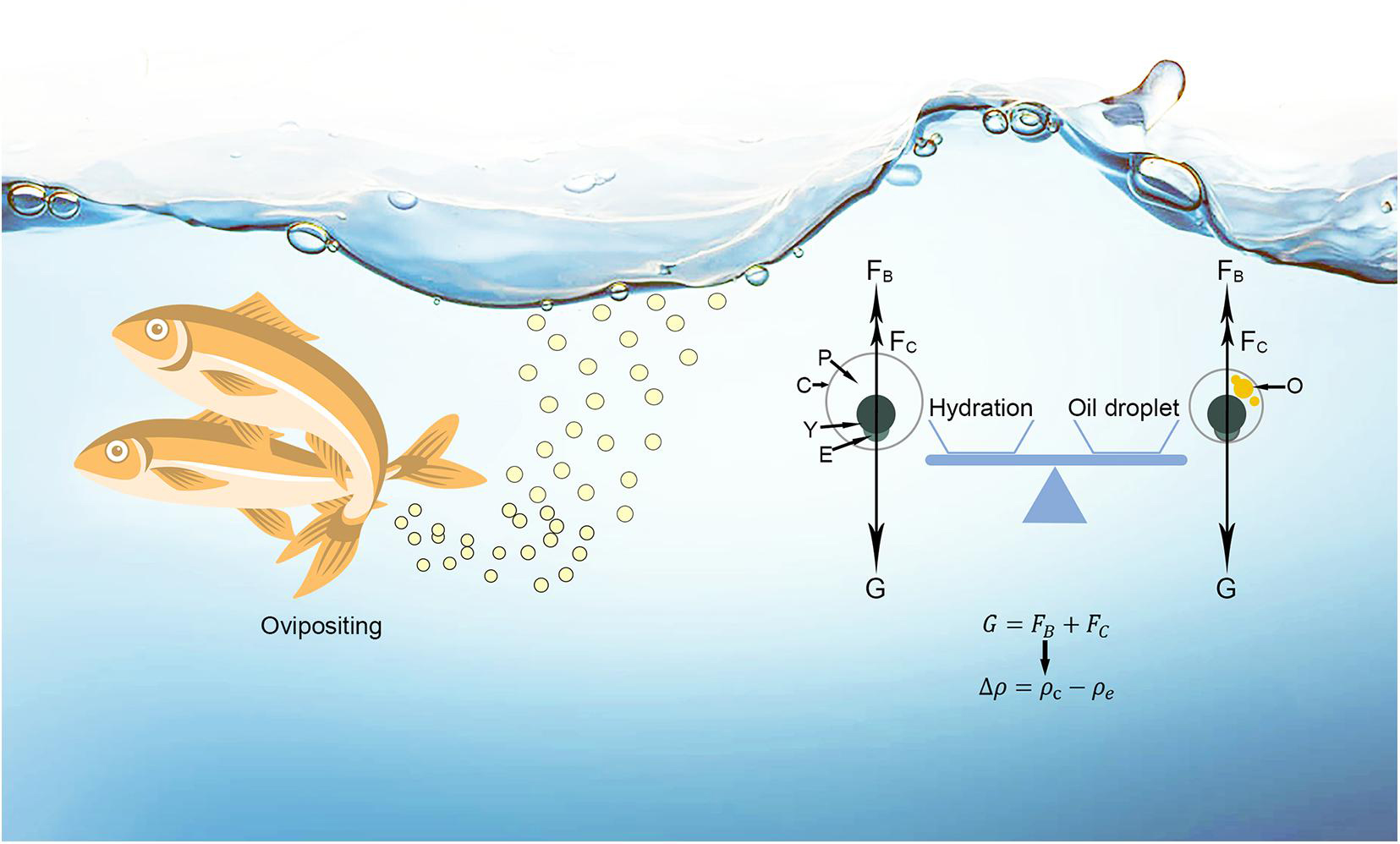
Figure 1. A schematic diagram of freshwater pelagic eggs after oviposition. G, gravity; FB, buoyant force; FC, current force; P, perivitelline space; C, chorion; Y, yolk; E, embryo; O, oil droplet; △ρ, buoyancy; ρe and ρc are the densities of egg and current.
Phylogenetic Analysis
Using mitochondrial DNA sequences from GenBank, the cytochrome b (Cyt b) gene sequences for 48 species of freshwater fishes with pelagic eggs were collated, including Perciformes (16 species), Clupeiformes (3 species), Cypriniformes (15 species), Siluriformes (2 species) and Characiformes (12 species) (accession numbers of all the sequences are listed in Supplementary Table 1). Multiple alignments of sequences were performed with MUSCLE (Edgar, 2004). Ambiguous regions of alignments were removed in batches using Gblocks v.0.91 (Talavera and Castresana, 2007). Substitution saturation of sequence datasets was tested with DAMBE (Xia, 2013). Because the index of substitution saturation (Iss) was significantly lower than the critical index of substitution saturation (Iss.c), the sequence dataset was little saturated and appropriate for phylogenetic analysis. ModelFinder was used to choose optimal models in PhyloSuite v1.2.2 (Kalyaanamoorthy et al., 2017; Zhang et al., 2020). The best-fit model GTR + I + G was selected using the Bayesian Information Criterion (BIC). MrBayes 3.2.6 was used to calculate posterior probabilities of recovered clades with the best-fit model (Ronquist et al., 2012). Markov chains, one cold and three heated, were run simultaneously for 2,000,000 generations. In the initial sample, trees were saved every 200 generations for a total size of 10,000. Graphical inspection of tree log-likelihood indicated that stationarity was reached within 200,000 generations, with the first 200,000 generations (1,000 sampled trees) subsequently discarded as burn-in, and the remaining 1,800,000 generations (9,000 sampled trees) used in subsequent analysis. For the 9,000 remaining trees, a majority rule consensus tree was calculated and used to determine the posterior probabilities of clades. Maximum likelihood phylogenies were performed using IQ-TREE v.1.6.8 (Nguyen et al., 2015) under the model automatically selected by IQ-TREE with 200,000 ultrafast bootstraps as well as the Shimodaira–Hasegawa–like approximate likelihood-ratio test (Guindon et al., 2010). The topologies of phylogenetic trees obtained by Maximum likelihood and Bayesian inference analyses were consistent (Supplementary Figure 1).
Phylogenetic Regression
We used phylogenetic regression (R package phylolm 2.6.2; Ho et al., 2016) to fit two models to the data. The first modelled the probability that a species produced pelagic eggs containing oil droplets (>5% of egg volume) as a function of the proportional increase in size of the perivitelline space after hydration. In the second model, the probability of oil droplets was modelled as a function of mode of parental care (nonguarder vs. guarder) and habitat flow regime (lentic vs. lotic). Both models were fitted using a Bernoulli GLM with logit link function and controlled for non-independence between species due to phylogenetic relatedness (Paradis and Claude, 2002). All explanatory covariates could not be included in a single model due to collinearity.
Results
In freshwaters, the pelagic eggs of bony fishes achieve a balance between the forces of gravity, buoyancy and flow to remain in suspension (Figure 1), summarised as:
Where G is gravity, FB is buoyant force, and FC is the sum of vertical forces generated by current flow. FC is derived from water flow, including the effects of drag and flow turbulence (Garcia, 2008; Jia et al., 2020). FC is variable, since it depends on local hydrology (Garcia et al., 2013, 2015; Liu et al., 2019). As the force patterns of fish eggs are concordant in seawater and freshwater, egg buoyancy is equal to the difference between the densities of water current and the egg when the pelagic egg remains in suspension. Based on Sundby and Kristiansen (2015), the buoyancy (△ρ) of freshwater pelagic eggs can be expressed as:
Where ρe, ρc, ρem+y, ρp, ρo and ρch are the densities of egg, water current, embryo and yolk, perivitelline space, oil droplet and chorion; while V represents the volume of each of these elements. It is known that ρo and ρp (0.926 kg l–1 and 0.998 kg l–1, respectively) are lower than ρemy and ρch (1.259 kg l–1 and 1.204 kg l–1, respectively) (Sundby and Kristiansen, 2015; Baras et al., 2018). When ρc is constant, △ρ can be increased by reducing ρe. Thus, in order for an egg to remain in the water column, selection must reduce egg density either through increasing Vo or Vp, or both.
Pelagic eggs of the Anostomidae, Characidae, Prochilodontidae (Characiformes), Cobitidae, Cyprinidae (Cypriniformes) and Pimelodidae (Siluriformes) all exhibited large perivitelline space volumes (mean of 93.5% of total egg volume), but lacked an oil droplet (Figure 2). Pelagic eggs of the Clupeidae (Clupeiformes), Hiodontidae (Osteoglossiformes), Percichthyidae and Terapontidae (Perciformes) also showed a relatively large perivitelline space (mean of 89.3% of total egg volume), but with a small oil droplet (average 0.4% of egg volume). In the Pantodontidae (Osteoglossiformes), Anabantidae, Channidae, Latidae, Osphronemidae, Sciaenidae (Perciformes) and Mastacembelidae (Synbranchiformes), oil droplets comprised over 10% of total egg volume (Figure 2). Threshold analyses show that in the 55 species of freshwater pelagic eggs for which data were available, buoyancy varied from −0.140 to −0.040 kg l–1 (Figure 3; Supplementary Table 1).
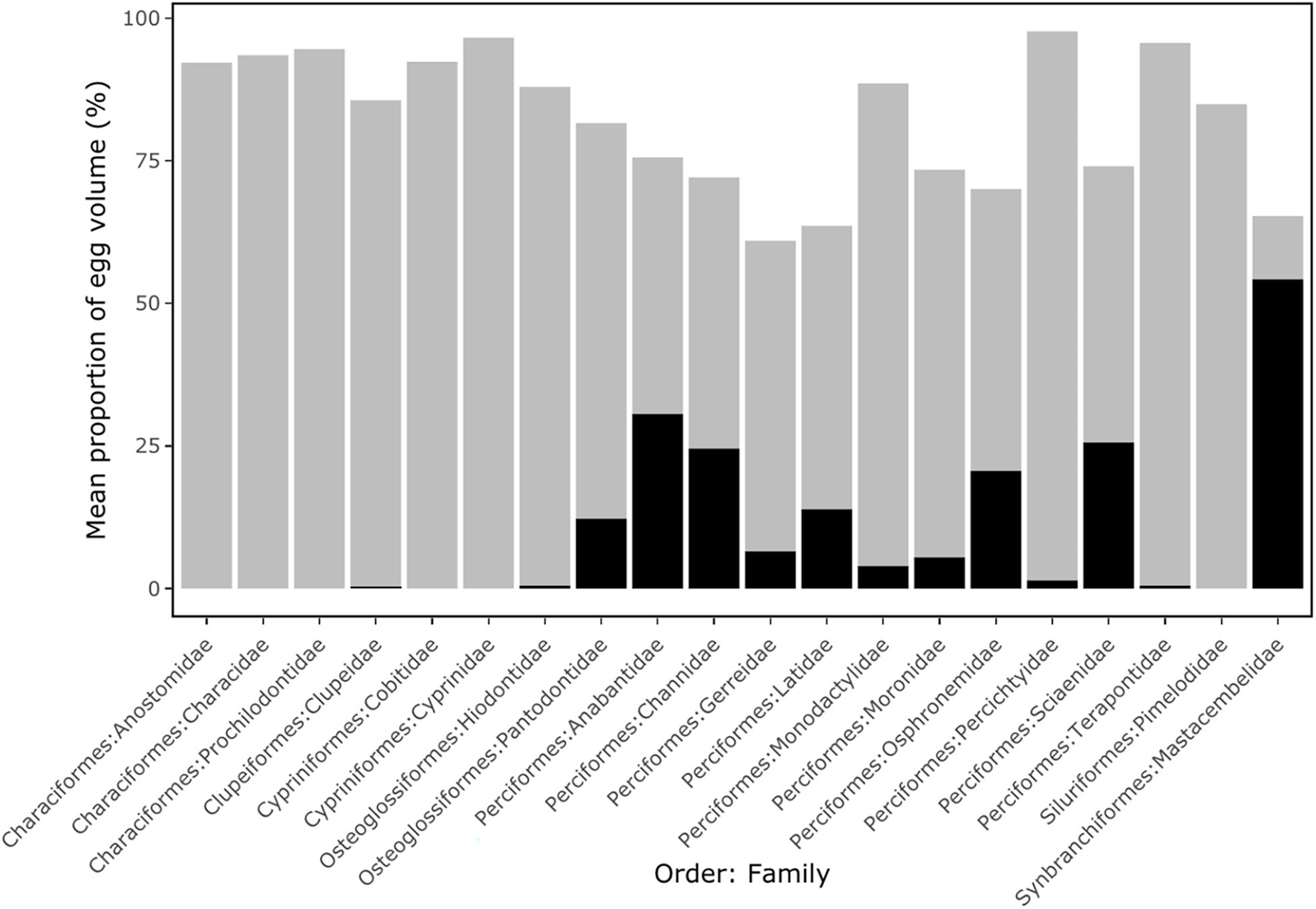
Figure 2. The mean proportion of egg volume occupied by oil droplet (black columns) and perivitelline space (grey columns) split by Order and Family for 55 species of freshwater fish with pelagic eggs. Details are shown in Supplementary Table 1.
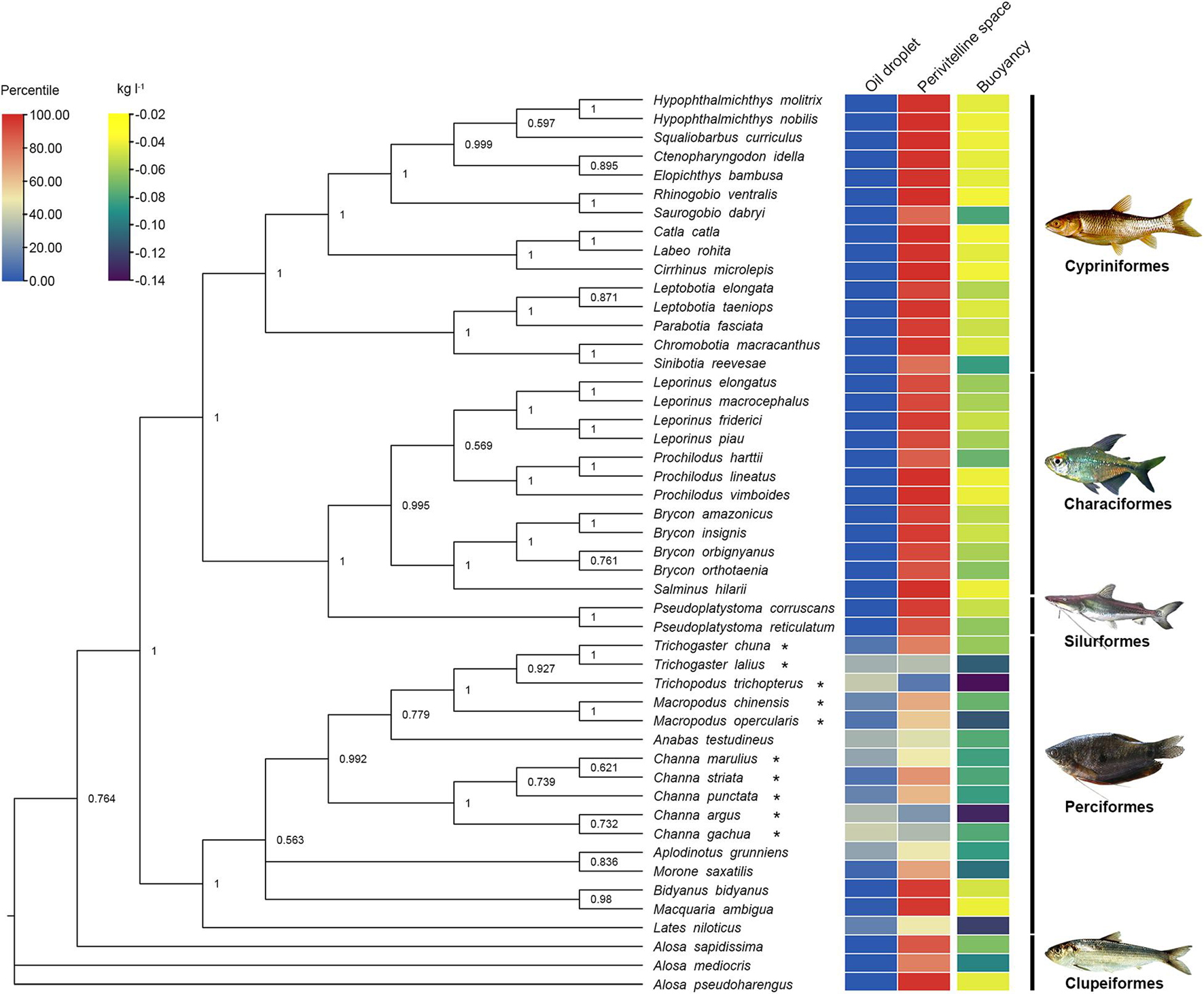
Figure 3. Phylogenetic relationships of 48 species of freshwater fishes with pelagic eggs showing oil droplet (OG), perivitelline space (PS), buoyancy (△ρ) (Supplementary Table 1). Colours from blue to red indicate the range of the proportion of oil droplet and perivitelline space from 0.00 to 100.00%; colours from purple to yellow represent the range of buoyancy values from −0.14 to −0.02 kg l–1. The tree was constructed with Bayesian analysis of cytochrome b gene dataset based on GTR+I+G model. Numbers at nodes represent posterior possibilities over 0.5. Asterisks indicate reproductive guild of aphrophils. Fish images are from FishBase (https://www.fishbase.se/search.php).
These patterns of perivitelline space and oil droplet size show clear phylogenetic associations (Figure 3). In the Orders of Characiformes, Siluriformes, Cypriniformes and Clupeiformes, pelagic eggs exhibit the same reproductive trait of a large perivitelline space, while the pelagic eggs of the Perciformes possess a large oil droplet. The buoyancy of most perciform fishes is lower than that of other taxa.
Phylogenetic regression showed a significant negative association between the relative size of the perivitelline space and the probability of oil droplets in the egg (Table 1 and Figure 4). There was also a significant negative association between the probability of possessing oil droplets and occupancy of lotic habitats, and a positive association with performance of parental care of eggs (Table 2).
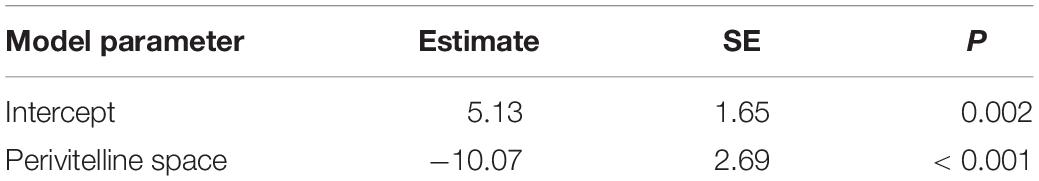
Table 1. Summary of a Bernoulli GLM to model the probability of oil droplets in the pelagic eggs of freshwater fishes as a function of relative size of perivitelline space, modelled using phylogenetic regression.
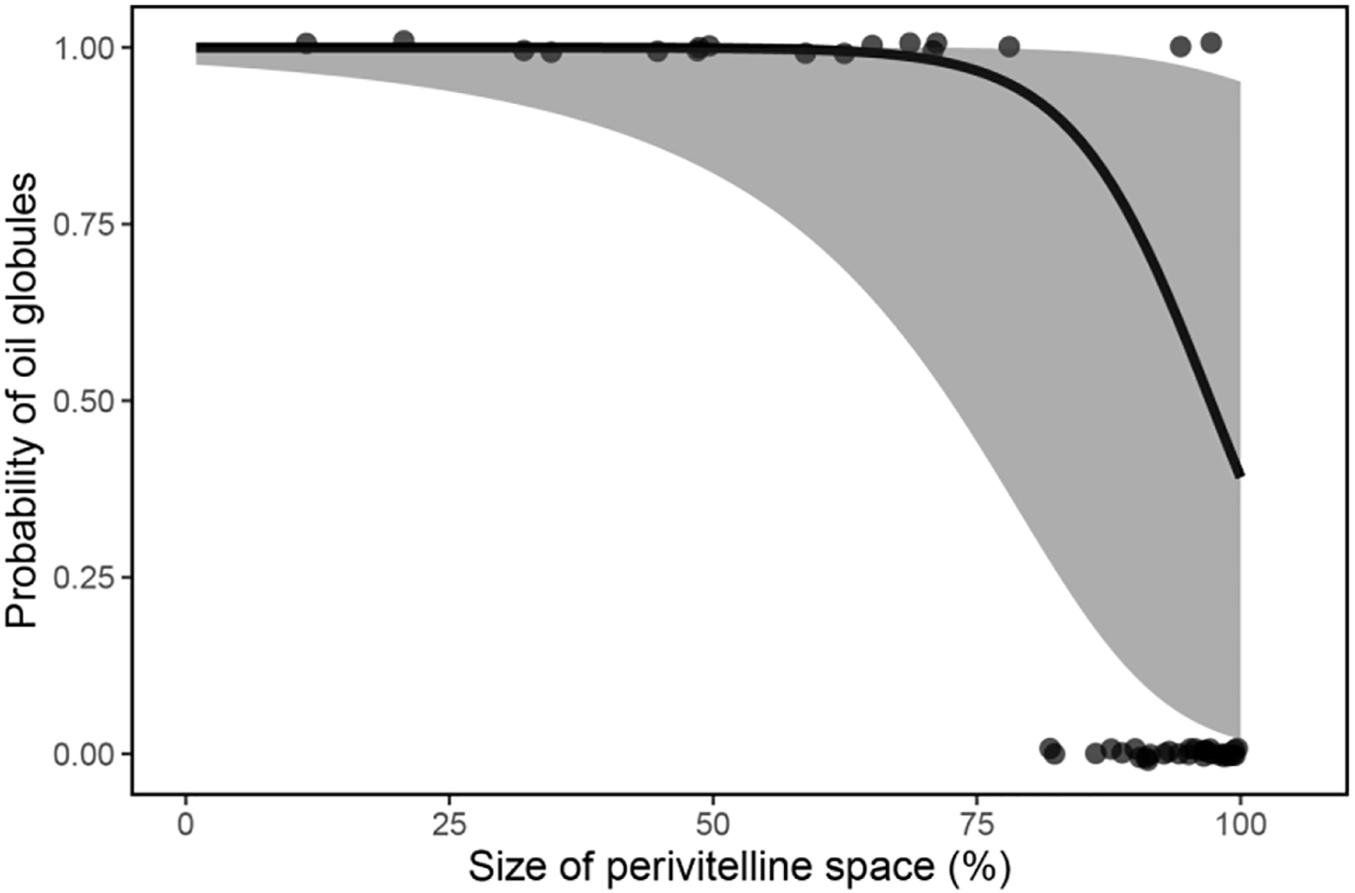
Figure 4. Mean fitted probability (solid line) of fish possessing oil globules in their eggs as a function of the relative size of perivitelline space (%) with 95% confidence intervals (shaded area). Data were modelled by phylogenetic regression with a Bernoulli GLM.
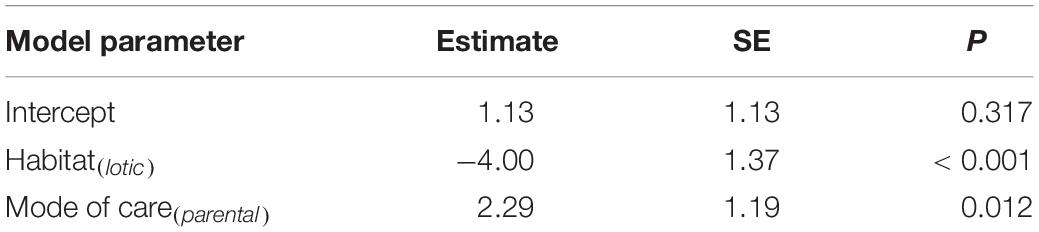
Table 2. Summary of a Bernoulli GLM to model the probability of oil droplets in the pelagic eggs of freshwater fishes as a function of habitat and mode of care modelled using phylogenetic regression.
Discussion
In freshwater teleost fishes with pelagic eggs, a general assumption is that egg buoyancy is achieved through the presence of low-density oil droplets in the egg. However, the majority of these species either do not possess oil droplets in their eggs, or the relative size of oil droplets is too small to effectively provide buoyancy (Baras et al., 2018). A model of egg buoyancy showed that close to neutral buoyancy could be achieved in freshwater through an increased volume of oil droplets (Vo) or perivitelline space (Vp). However, because the density of the perivitelline space (ρp) approximately matches current density (ρc), the perivitelline space of pelagic eggs is capable of generating near-neutral buoyancy (“semi-buoyancy”) in lotic habitats.
The pelagic eggs of Characiformes, Clupeiformes, Cypriniformes, Hiodontidae (Osteoglossiformes), Percichthyidae, Terapontidae (Perciformes) and Siluriformes exhibited a large perivitelline space volume, with either no oil droplet or a small oil droplet present. This large perivitelline space is associated with hydration and reduced egg density, though because the osmolality of eggs is higher than that of freshwater, a large perivitelline space alone cannot generate neutral buoyancy in freshwater. However, the energetic cost of hydration is lower than that of sequestering lipid in eggs, which makes a limited contribution to embryonic tissue formation (Wiegand, 1996; Iwamatsu et al., 2008; Baras et al., 2018). Because most species with pelagic eggs and a large perivitelline space are associated with high-flow habitats, typical of the middle and upper reaches of rivers (Table 2, Supplementary Table 1), eggs remain suspended in the water column because the vertical forces generated by water current flow (FC). Oil droplets in the eggs of Pantodontidae (Osteoglossiformes), Anabantidae, Channidae, Latidae, Osphronemidae, Sciaenidae (Perciformes) and Mastacembelidae (Synbranchiformes) comprise over 10% of total egg volume, which thereby contributes significantly to buoyancy. In these taxa spawning is associated with slow-flowing or lentic habitats (Table 2 and Supplementary Table 1). Because the density of oil droplets is lower than freshwater, eggs can achieve positive buoyancy if they possess a relatively large oil droplet when FC is low or zero, but at the cost of sequestering lipid in eggs.
These general conclusions from the buoyancy model were supported by phylogenetic regression analysis. We detected a significant negative association between the relative size of the perivitelline space of freshwater pelagic eggs and probability of possessing oil droplets that exceeded 5% of egg volume (Table 1). This association implies that eggs either possess a large volume of oil droplets or a large perivitelline space, but not both. The association also suggests a trade-off in these two buoyancy tactics, possibly related to the energetic costs of using energetically expensive oil droplets for buoyancy. It is striking that the volume of oil droplets of marine pelagic eggs never exceeds 9% of total egg volume and averages approximately 2% (Baras et al., 2018). Because the osmolarity of marine eggs is lower than the osmolarity of seawater, but is higher than that of ovarian fluid (Finn et al., 2002; Wootton and Smith, 2015; Sørensen et al., 2016), it appears more adaptive to achieve buoyancy through hydration in the ovary, presumably because of the energetic cost associated with allocating lipid to eggs.
Given that reproduction in fish is energetically costly, it is assumed that energy is allocated among reproductive roles under strong selective forces (Harshman and Zera, 2007; McBride et al., 2015; Robertson and Collin, 2015; Muller et al., 2019). The physiological processes associated with oil droplet formation and hydration are recognized as energy demanding processes (Marshall et al., 1999; Riis-Vestergaard, 2002; Cerdà et al., 2007; Martin et al., 2017). Hence the negative association between the possession of oil droplets and size of perivitelline space in pelagic freshwater eggs is assumed to represent a bio-energetic trade-off (Morrongiello et al., 2012; Closs et al., 2013; Dani and Kodandaramaiah, 2017). The model of buoyancy (△ρ) (Equation 2) suggests that eggs must achieve a buoyancy that exceeds −0.14 kg l–1 (Supplementary Table 1). Although the threshold value of −0.14 kg l–1 may not be the minimum for freshwater pelagic eggs, but it was the lowest estimate in the species for which data were available and may represent a lower limit for functional buoyancy in freshwater. The adaptive significance for this threshold value is not clear, but presumably serves to maintain an egg above the substrate sufficiently to prevent abrasive damage. Although egg density (ρe) is always higher than current density (ρc), freshwater eggs can approach neutral buoyancy in flowing water when these two parameters are similar in magnitude.
We detected a significant negative association between the presence of large oil droplets and occupancy of lotic habitats (Table 2). This finding supports the prediction, derived from Equation 2, that hydration can function as an effective means of buoyancy or “semi-buoyancy” if eggs experience water flow, but not in lentic habitats where buoyancy can only be achieved by allocating low-density lipids to eggs. The results indicate the evolution of ARTs in pelagic freshwater fish eggs with large oil droplets and a large perivitelline space is environment dependent. This finding supports the view that the expression of ARTs in many species is influenced by environmental effects, including relative competitive ability (Foote, 1990), population parameters (Kodric-Brown, 1986) and ecological cues (Hamilton et al., 2006).
Phylogenetic regression analysis also showed that the presence of large oil globules was associated with freshwater species that perform parental care (Table 2). This finding is a logical outcome of a proposed incompatibility between care of pelagic eggs in lotic habitats; the wide dispersal of eggs exposed to a strong current means that the opportunity for parental care is limited. This finding also implies an extra cost associated with the provision of parental care in addition to the costs typically associated with parental care in fishes (Smith and Wootton, 1995; Baldridge and Lodge, 2013), through the allocation of oil droplets to eggs to facilitate buoyancy. Since many fish species display parental care, the mode of parental care probably influences the evolution of ARTs. Taborsky (1994, 1999) demonstrated that all possible patterns of parental care, including no care, uniparental, biparental, multiparent and alloparental care might in turn affect the variability of ARTs in fish.
The pelagic eggs of the Characiformes, Siluriformes, Cypriniformes and Clupeiformes are associated with fast-flowing conditions (Supplementary Table 1), and these Orders appear to have evolved the same reproductive trait of a large perivitelline space. However, the pelagic eggs of the Perciformes are typically spawned in slow-flowing freshwater (Supplementary Table 1), and instead possess a large oil droplet. Although the buoyancy of a majority of perciform fishes is lower, the reproductive pattern for most of these fishes is of aphrophils, with eggs deposited in bubble nests, which typically comprise a floating raft of mucous foam that keeps the eggs at the surface while guarded by a parent (Balon, 1975; Hostache and Mol, 1998; Wootton and Smith, 2015). These egg buoyancy adaptations suggest the evolution of ARTs in contrasting biotopes, which potentially contributes to reproductive isolation (Taylor, 1999; Funk et al., 2006; Reznick et al., 2021).
Conclusion
We applied an egg buoyancy model, combined with phylogenetic regression, to understand the contribution of oil droplets and hydration to the buoyancy of pelagic eggs of freshwater teleosts. Our analysis showed that egg buoyancy is achieved with a large perivitelline space or large volume of oil droplets under different selective conditions, which potentially reflects a trade-off in energy allocation. Phylogenetic regression showed that a large perivitelline space was associated with lotic environments in which semi-buoyancy of eggs can be achieved without incurring energetic costs associated with allocating lipid to eggs. Freshwater fishes with parental care were associated with eggs that possessed a large volume of oil droplets in lentic habitats. These findings demonstrate alternative tactics for buoyancy in freshwater fish eggs, and offer a novel insight into the evolution of ARTs in teleost fishes in contrasting biotopes.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
PX, JC, and FC designed the research. FC, CS, YW, JH, WX, and GX collected and analysed the data. FC, CS, PX, and JC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB31000000) and the State Key Laboratory of Freshwater Ecology and Biotechnology (Grant No. 2019FBZ03).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Andrea E. A. Stephens, Jiarui Liu, and Martin Reichard for constructive advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.736718/full#supplementary-material
Footnotes
References
Baldridge, A. K., and Lodge, D. M. (2013). Intraguild predation between spawning smallmouth bass (Micropterus dolomieu) and nest-raiding crayfish (Orconectes rusticus): implications for bass nesting success. Fresh. Biol. 58, 2355–2365. doi: 10.1111/fwb.12215
Balon, E. K. (1975). Reproductive guilds of fishes: a proposal and definition. J. Fish. Board Can. 32, 821–864. doi: 10.1139/f75-110
Baras, E., Arifin, O. Z., Slembrouck, J., Subagja, J., Kristanto, A. H., and Legendre, M. (2018). Oil globule size in fish eggs: a matter of biome and reproductive strategy. Fish Fish. 19, 996–1002. doi: 10.1111/faf.12307
Cerdà, J., Fabra, M., and Raldúa, D. (2007). “Physiological and molecular basis of fish oocyte hydration,” in The Fish Oocyte, eds P. Babin, J. Cerdà, and E. Lubzens (Dordrecht: Springer), 349–396.
Closs, G. P., Hicks, A. S., and Jellyman, P. G. (2013). Life histories of closely related amphidromous and non-migratory fish species: a trade-off between egg size and fecundity. Fresh. Biol. 58, 1162–1177. doi: 10.1111/fwb.12116
Craik, J. C. A., and Harvey, S. M. (1987). The causes of buoyancy in eggs of marine teleosts. J. Mar. Biol. Assoc. UK 67, 169–182. doi: 10.1017/S0025315400026436
Dani, K. G. S., and Kodandaramaiah, U. (2017). Plant and animal reproductive strategies: lessons from offspring size and number tradeoffs. Front. Ecol. Evol. 5:38. doi: 10.3389/fevo.2017.00038
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Fabra, M., Raldúa, D., Power, D. M., Deen, P. M., and Cerda, J. (2005). Marine fish egg hydration is aquaporin-mediated. Science 307, 545–545. doi: 10.1126/science.1106305
FAO (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals. Rome. Licence: CC BY-NC-SA 3.0 IGO. Rome: FAO.
Finn, R. N., Østby, G. C., Norberg, B., and Fyhn, H. J. (2002). In vivo oocyte hydration in Atlantic halibut (Hippoglossus hippoglossus); proteolytic liberation of free amino acids, and ion transport, are driving forces for osmotic water influx. J. Exp. Biol. 205, 211–224.
Foote, C. J. (1990). An experimental comparison of male and female spawning territoriality in a pacific salmon. Behaviour 115, 283–314. doi: 10.1163/156853990X00617
Funk, D. J., Nosil, P., and Etges, W. J. (2006). Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc. Natl. Acad. Sci. U.S.A. 103, 3209–3213. doi: 10.1073/pnas.0508653103
Garcia, M. (2008). Sedimentation Engineering: Processes, Measurements, Modeling, and Practice. Reston: American Society of Civil Engineers Publications. Reston VA: American Society of Civil Engineers Publications.
Garcia, T., Jackson, P. R., Murphy, E. A., Valocchi, A. J., and Garcia, M. H. (2013). Development of a fluvial egg drift simulator to evaluate the transport and dispersion of Asian carp eggs in rivers. Ecol. Modell. 263, 211–222. doi: 10.1016/j.ecolmodel.2013.05.005
Garcia, T., Zuniga Zamalloa, C., Jackson, P. R., Murphy, E. A., and Garcia, M. H. (2015). A laboratory investigation of the suspension, transport, and settling of silver carp eggs using synthetic surrogates. PLoS One 10:e0145775. doi: 10.1371/journal.pone.0145775
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Hamilton, I. M., Haesler, M. P., and Taborsky, M. (2006). Predators, reproductive parasites, and the persistence of poor males on leks. Behav. Ecol. 17, 97–107. doi: 10.1093/BEHECO/ARI099
Harshman, L. G., and Zera, A. J. (2007). The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86. doi: 10.1016/j.tree.2006.10.008
Ho, L. S. T., Ane, C., Lachlan, R., Tarpinian, K., Feldman, R., Yu, Q., et al. (2016). Package ‘phylolm’. Available online at: http://cran.r-project.org/web/packages/phylolm/index.Html. (accessed November 8, 2020).
Hoagstrom, C. W., and Turner, T. F. (2015). Recruitment ecology of pelagic-broadcast spawning minnows: paradigms from the ocean advance science and conservation of an imperilled freshwater fauna. Fish Fish. 16, 282–299. doi: 10.1111/faf.12054
Hostache, G., and Mol, J. H. (1998). Reproductive biology of the neotropical armoured catfish Hoplosternum littorale (Siluriformes-Callichthyidae): a synthesis stressing the role of the floating bubble nest. Aquat. Living Resour. 11, 173–185. doi: 10.1016/S0990-7440(98)80114-9
Iwamatsu, T., Muramatsu, T., and Kobayashi, H. (2008). Oil droplets and yolk spheres during development of Medaka embryos. Ichthyol. Res. 55, 344–348. doi: 10.1007/s10228-008-0048-z
Jia, W. F., Zhang, S. H., Yang, Y. F., and Yi, Y. J. (2020). A laboratory investigation of the transport mechanism of floating fish eggs: a case study of Asian carps. Aquaculture 519, 734855. doi: 10.1016/j.aquaculture.2019.734855
Kalyaanamoorthy, S., Bui Quang, M., Wong, T. K. F., von Haeseler, A., and Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Kamler, E. (2005). Parent–egg–progeny relationships in teleost fishes: an energetics perspective. Rev. Fish Biol. Fish. 15, 399–421. doi: 10.1007/s11160-006-0002-y
Kodric-Brown, A. (1986). Satellites and sneakers: opportunistic male breeding tactics in pupfish (Cyprinodon pecosensis). Behav. Ecol. Sociobiol. 19, 425–432. doi: 10.1007/BF00300545
Kunz-Ramsay, Y. (2013). Developmental Biology of Teleost Fishes. Dordrecht: Springer Science and Business Media.
Liu, X., Lin, J., Peng, Q., Yu, K., Chen, Y., and Zhuang, J. (2019). Experimental research on fish eggs’ movement using particle tracking velocimetry technique. J. Hyd. Eng. 49, 501–511.
Marshall, C. T., Yaragina, N. A., Lambert, Y., and Kjesbu, O. S. (1999). Total lipid energy as a proxy for total egg production by fish stocks. Nature 402, 288–290. doi: 10.1038/46272
Martin, B. T., Heintz, R., Danner, E. M., and Nisbet, R. M. (2017). Integrating lipid storage into general representations of fish energetics. J. Anim. Ecol. 86, 812–825. doi: 10.1111/1365-2656.12667
McBride, R. S., Somarakis, S., Fitzhugh, G. R., Albert, A., Yaragina, N. A., Wuenschel, M. J., et al. (2015). Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish. 16, 23–57. doi: 10.1111/faf.12043
Monroe, M. J., Amundsen, T., Utne-Palm, A. C., and Mobley, K. B. (2016). Seasonal variation in male alternative reproductive tactics. J. Evol. Biol. 29, 2362–2372. doi: 10.1111/jeb.12981
Morrongiello, J. R., Bond, N. R., Crook, D. A., and Wong, B. B. (2012). Spatial variation in egg size and egg number reflects trade-offs and bet-hedging in a freshwater fish. J. Anim. Ecol. 81, 806–817. doi: 10.1111/j.1365-2656.2012.01961.x
Mueller, J. S., Cheek, B. D., Chen, Q., Groeschel, J., Brewer, S. K., and Grabowski, T. B. (2013). A simple device for measuring the minimum current velocity to maintain semi-buoyant fish eggs in suspension. Proc. Nat. 45, 84–89.
Muller, E. B., Lika, K., Nisbet, R. M., Schultz, I. R., Casas, J., Gergs, A., et al. (2019). Regulation of reproductive processes with dynamic energy budgets. Funct. Ecol. 33, 819–832. doi: 10.1111/1365-2435.13298
Nguyen, L. T., Schmidt, H. A., Von Haeseler, A., and Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. doi: 10.1093/molbev/msu300
Oliveira, R. F., Taborsky, M., and Brockmann, H. J. (2008). Alternative Reproductive Tactics. New York, NY: Cambridge University Press.
Paradis, E., and Claude, J. (2002). Analysis of comparative data using generalized estimating equations. J. Theor. Biol. 218, 175–185. doi: 10.1006/jtbi.2002.3066
Reznick, D. N., Travis, J., Pollux, B. J. A., and Furness, A. I. (2021). Reproductive mode and conflict shape the evolution of male attributes and rate of speciation in the fish family Poeciliidae. Front. Ecol. Evol. 9:639751. doi: 10.3389/fevo.2021.639751
Riis-Vestergaard, J. (2002). Energy density of marine pelagic fish eggs. J. Fish Biol. 60, 1511–1528. doi: 10.1111/j.1095-8649.2002.tb02444.x
Robertson, D. R., and Collin, R. (2015). Inter- and Intra-specific variation in egg size among reef fishes across the Isthmus of Panama. Front. Ecol. Evol. 2:84. doi: 10.3389/fevo.2014.00084
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Smith, C., and Wootton, R. J. (1995). The costs of parental care in teleost fishes. Rev. Fish Biol. Fish. 5, 7–22. doi: 10.1007/BF01103363
Smith, T. B., and Skúlason, S. (1996). Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 27, 111–133. doi: 10.1146/annurev.ecolsys.27.1.111
Sørensen, S. R., Butts, I. A. E., Munk, P., and Tomkiewicz, J. (2016). Effects of salinity and sea salt type on egg activation, fertilization, buoyancy and early embryology of European eel, Anguilla anguilla. Zygote 24, 121–138. doi: 10.1017/S0967199414000811
Stiver, K. A., Harris, R. M., Townsend, J. P., Hofmann, H. A., and Alonzo, S. H. (2015). Neural gene expression profiles and androgen levels underlie alternative reproductive tactics in the ocellated wrasse, Symphodus ocellatus. Ethology 121, 152–167. doi: 10.1111/eth.12324
Sundby, S., and Kristiansen, T. (2015). The principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. PLoS One 10:e0138821. doi: 10.1371/journal.pone.0138821
Taborsky, M. (1994). Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv. Study Behav. 23, 1–100. doi: 10.1016/S0065-3454(08)60351-4
Taborsky, M. (1999). “Conflict or cooperation: what determines optimal solutions to competition in fish reproduction?,” in Behaviour and Conservation of Littoral Fishes, eds R. F. Oliveira, V. Almada, and E. Gonçalves (Lisbon: Instituto Superior de Psicologia Aplicada), 301–349.
Taborsky, M. (2008). “Alternative reproductive tactics in fish,” in Alternative Reproductive Tactics, eds R. F. Oliveira, M. Taborsky, and H. J. Brockmann (Cambridge: Cambridge University Press), 251–299.
Taborsky, M., and Brockmann, H. J. (2010). “Alternative reproductive tactics and life history phenotypes,” in Animal Behaviour: Evolution and Mechanisms, ed. P. Kappeler (Berlin: Springer), 537–586.
Talavera, G., and Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577. doi: 10.1080/10635150701472164
Taylor, E. B. (1999). Species pairs of north temperate freshwater fishes: evolution, taxonomy, and conservation. Rev. Fish Biol. Fish. 9, 299–324. doi: 10.1023/A:1008955229420
Thorsen, A., Kjesbu, O. S., Fyhndr, H. J., and Solemdal, P. (1996). Physiological mechanisms of buoyancy in eggs from brackish water cod. J. Fish Biol. 48, 457–477. doi: 10.1111/j.1095-8649.1996.tb01440.x
West-Eberhard, M. J. (1986). Alternative adaptations, speciation, and phylogeny. Proc. Natl. Acad. Sci. U.S.A. 83, 1388–1392. doi: 10.1073/pnas.83.5.1388
Wiegand, M. D. (1996). Composition, accumulation and utilization of yolk lipids in teleost fish. Rev. Fish Biol. Fish. 6, 259–286. doi: 10.1007/BF00122583
Wootton, R. J. (1994). Life histories as sampling devices: optimum egg size in pelagic fishes. J. Fish Biol. 45, 1067–1077. doi: 10.1111/j.1095-8649.1994.tb01073.x
Wootton, R. J., and Smith, C. (2015). Reproductive Biology of Teleost Fishes. Oxford: John Wiley & Sons, Inc.
Xia, X. (2013). DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 30, 1720–1728. doi: 10.1093/molbev/mst064
Keywords: alternative reproductive tactic, lipid, model, parental care, perivitelline space, phylogenetic regression
Citation: Chen F, Smith C, Wang Y, He J, Xia W, Xue G, Chen J and Xie P (2021) The Evolution of Alternative Buoyancy Mechanisms in Freshwater Fish Eggs. Front. Ecol. Evol. 9:736718. doi: 10.3389/fevo.2021.736718
Received: 05 July 2021; Accepted: 06 September 2021;
Published: 28 September 2021.
Edited by:
Mingbo Yin, Fudan University, ChinaReviewed by:
Alex Cordoba-Aguilar, National Autonomous University of Mexico, MexicoJack Chi-Ho Ip, Hong Kong Baptist University, Hong Kong, SAR China
Copyright © 2021 Chen, Smith, Wang, He, Xia, Xue, Chen and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chen, chenjun@ihb.ac.cn; Ping Xie, xieping@ihb.ac.cn
 Feng Chen
Feng Chen Carl Smith3,4
Carl Smith3,4  Ping Xie
Ping Xie