Permanent cardiac pacing is a standard, reliable and widely accessible method of bradycardia treatment. Since the first implantable pacemaker was developed in 1959 by Rune Elmqvist, cardiac pacing has undergone a dynamic technological revolution.1
Pacemaker and lead technology have developed rapidly, and modern pacemakers are now automatic and more reliable. Epicardial leads have been replaced by transvenous leads and pacemakers are programmed to sense underlying cardiac activity and provide pacing only when needed.
The increased life expectancies of the steadily growing elderly population have led to increased permanent pacemaker (PPM) implantation rates and new challenges in treating bradycardia.2
Even though PPMs brought indisputable benefits to patients with bradycardia, the constantly rising standards for patient wellbeing and better patient follow-up have revealed patients who fail to tolerate conventional right ventricular (RV) pacing.3–10
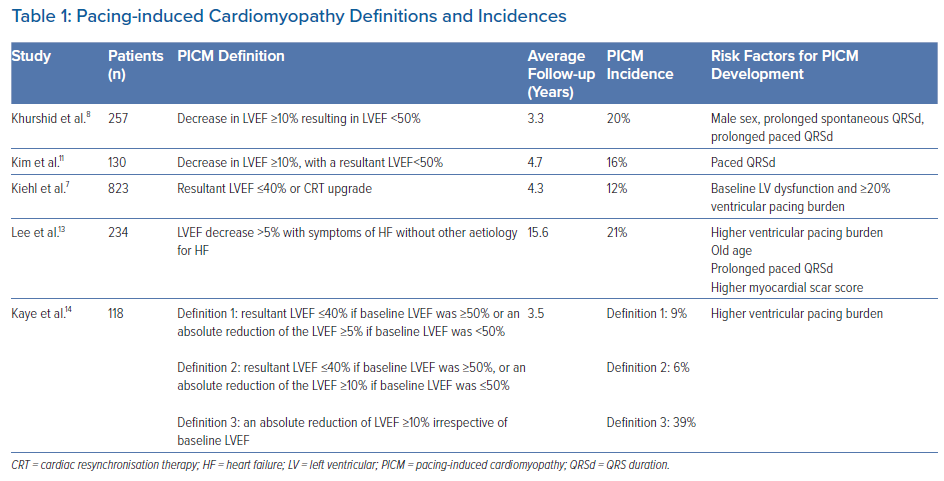
In some of them, left ventricular ejection fraction (LVEF) can decline after pacing. This condition is known as pacing-induced cardiomyopathy (PICM). The current literature has several working sets of diagnostic criteria for identifying PICM, primarily based on changes in the LVEF (Table 1). The authors’ review of published research found these were the four most frequent PICM definitions:
- Decreased LVEF by 10% or more or below 50% regardless of patient symptoms;8,11
- Decreased LVEF below 45% or a decline in LVEF that is greater than 10% after PPM implantation;12
- Decreased LVEF below 40% or an indication to CRT upgrade;7
- Decreased LVEF by 5% or more with heart failure (HF) symptoms with no other aetiology of HF.13
The literature estimates that 6–22% of all patients undergoing PPM implantation fulfil the criteria for PICM within 3–16 years.7,8,11 This wide range of prevalence is associated with differences in PICM definitions, the variability of the studied populations and variable lengths of follow-up.14
Moreover, there is a rising awareness that, at least in some patients, permanent RV pacing can lead to symptoms of HF without significant changes in LVEF, a condition called PICM syndrome.15
As shown recently, HF can often appear in a timescale of months, not years, after PPM implantation. A Danish national registry-based study including almost 28,000 patients undergoing PPM implantation found that almost 11% of them manifested with HF. This was significantly more than in the control group of patients without PPM, and most of these events occurred within 6 months of PPM implantation.10
Pathophysiology of Pacing-induced Cardiomyopathy
Physiological heart activation preserves atrioventricular (AV) and intraventricular conduction via the heart’s conduction system. This mechanism preserves AV synchrony and synchronous ventricular contraction.
The velocity of electrical signal transmission in Purkinje fibres is in a range of 2–4 m/s, as opposed to 0.4–0.8 m/s in ventricular muscle cells.16 RV pacing bypasses the physiological pathway, leading to slow myocyte-to-myocyte signal transmission, with a single electrical signal breakthrough in the RV apex or septum (depending on the stimulation site).
This results in disproportional RV and, more importantly, left ventricular (LV) mechanical and electrical activation, with the initial depolarisation occurring at the pacing site followed by delayed depolarisation of remote LV segments.17 These consequences of RV pacing are generally regarded as electro-mechanical ventricular dyssynchrony.
Different types of ventricular dyssynchrony are recognisable, i.e. interventricular (between the right and left ventricles) and intraventricular (within the right and left ventricles) electro-mechanical dyssynchrony.
Interventricular dyssynchrony can be measured using conventional Doppler echocardiographic imaging as the difference between the opening times of the pulmonary and aortic valves, i.e., aortopulmonary ejection delay.18,19 Intraventricular LV dyssynchrony is understood as a delay of mechanical activation between the various LV segments. It can be assessed using tissue Doppler imaging (TDI), or 2D speckle-tracking strain analysis, and real-time 3D echocardiography.20
The relationship between LV dyssynchrony and RV apical pacing was first shown in 2006 by Tops et al. in patients with AF treated with PPM implantation and subsequent AV nodal ablation. Here, LV dyssynchrony, measured using TDI, developed in almost 50% of patients after a mean follow-up of 3.8 years. Patients with LV dyssynchrony had a significant decline in LVEF and worsened New York Heart Association (NYHA) scores; in patients without LV dyssynchrony, the LVEF remained unchanged, and the NYHA score improved.21
As was shown soon after, RV apical pacing results in dyssynchronous LV contractions immediately after the start of pacing, even in patients with structurally normal hearts, and the presence of mechanical ventricular dyssynchrony, caused by RV pacing, was identified as the critical determinant of the detrimental effect of RV pacing on LV function.22–26
The time difference between the activation of individual LV segments leads to structural alteration and asymmetrical remodelling. This results from asymmetrical workloads between early activated septal and late activated LV lateral wall segments, reducing the workload for the septum and increasing the workload for the LV lateral wall. This is followed by thinning of the septum and hypertrophy of late activated LV lateral wall segments.27 It has been reported that the efficiency of cardiac pump function (the amount of stroke work generated by a unit of oxygen consumed) is approximately 30% lower in dyssynchronous than in synchronous hearts.28
As a result of non-physiological RV pacing, changes in ventricular blood perfusion, neurohumoral innervation and fatty acid metabolism have also been observed. Moreover, dyssynchrony results in changes in local myocardium oxygen demand. Different effective workloads of particular ventricular segments also cause changes in segmental myocardial perfusion and regional myocardial perfusion defects, even in the absence of coronary artery disease (CAD).29,30
Moreover, cardiac pacing has been associated with increased noradrenaline levels in myocardial tissue; in clinical research, early activated LV segments have been associated with a redistribution of sympathetic activity that resulted in regional LV defects of 123I-MIBG uptake.31,32
Altered myocardial metabolism can contribute to fibrosis, myofibrillar disarray and changes in cardiac extracellular matrix (ECM) metabolism. These changes are not detectable using conventional imaging methods, but they can be assessed in tissues and possibly also in the blood circulation.
RV apical pacing in dogs has been associated with asymmetrical hypertrophy of the late-activated lateral wall segments and led to ECM remodelling and overexpression of the collagen type II gene. Additionally, the lateral wall exhibited increased amounts of matrix-metalloproteinases (MMP), MMP-2, MMP-9, TIMP-1 and TIMP-3 expression.33
Adomian et al. identified myofibrillar disarray in nine out of 12 canine hearts after 3 months of RV apical pacing.34 Similar observations were confirmed in one of only a few clinical studies on histological changes following RV pacing in humans. In the study, chronic RV pacing led to myofibrillar hypertrophy, fatty depositions and the development of cardiac interstitial fibrosis.35
Risk Factors for Pacing-induced Cardiomyopathy and Dyssynchrony Assessment
There are known risk factors for PICM development in patients with frequent RV pacing, i.e., decreased pre-implant LVEF, older age, coronary artery disease (CAD) and wide spontaneous or paced QRS durations (QRSd).
According to recent research, the potentially harmful burden of RV pacing is lower (around 20%) than previously suggested by results from the MOST trial.5,7,13,14 The major problem in relying on these risk factors is their limited predictive value, so new and better methods for PICM risk assessment are needed.
As mentioned above, dyssynchronous LV ventricular contraction can be assessed using echocardiography, which has been shown to better identify patients at the highest risk of developing PICM.
Interventricular dyssynchrony, as a risk factor for PICM in patients with RV pacing, was assessed in the study by Bansal et al.23 This group demonstrated that patients with a significant aortopulmonary ejection delay (>40 ms) were more prone to developing a decrease in LVEF than patients with lower values. Multivariate analysis showed that significant interventricular dyssynchrony and a high burden of RV pacing were the only predictors of an LVEF decrease of >10%.23
RV pacing not only results in various forms of dyssynchrony but also affects LV function sooner than can be detected by LVEF measurements. As shown by Ahmad et al., LV function measured using global longitudinal strain (GLS) deteriorates much sooner compared to when it is measured using LVEF. Furthermore, the study showed that GLS values decline as soon as 1 month after the start of RV septal pacing, and the same patients had a decline in LVEF of ≥5% over 12 months of follow-up. In this study, lower values of GLS were an independent predictor of a decline in the LVEF during follow-up.36
Thus, the data show that echocardiography can be a valuable tool for risk stratification of PICM development. It can identify patients with dyssynchronous ventricular contractions due to RV pacing, which appear to be at the highest risk of a further decline in the LVEF. However, its use during the implant procedure is limited and, for that reason, new methods of dyssynchrony assessment are needed.
The traditional tool for non-invasive dyssynchrony assessment has been the surface 12-lead ECG. The most often used parameter of synchronous ventricular activation is the QRSd. This can be easily measured during implantation procedures and, for this reason, appears to be an ideal parameter for ventricular dyssynchrony assessment.
Although it has been shown in some studies that a wider paced QRSd is an independent multivariable predictor for PICM development, this has not been confirmed in other research.8,11,37,7,23 Its major limitation is that a conventional ECG visualises only the combined depolarisation of both ventricles and does not have the ability to assess their separate activation.38 QRS morphology offers more insight into ventricular activation patterns; however, this assessment is subject to significant error. Additionally, there are several definitions of left bundle branch block.39
Another ECG-derived parameter of dyssynchrony is the QRS area (QRSa).40 It is derived from orthogonal chest leads or calculated from a standard surface 12-lead ECG and converted to 3D vectorcardiography (Figure 1).41 The QRSa is an easily obtainable, reproducible parameter, which can be automatically calculated.42
Large QRS areas have been positively associated with volumetric responses to cardiac resynchronisation therapy (CRT) and are superior in predicting CRT responses over QRSd or QRS morphologies.43
In CRT patients, a decrease in the QRSa was an independent predictor of survival and reverse cardiac remodelling, especially in patients with larger baseline QRSa.44 Also in CRT patients, the QRSa has been shown to correlate better with LV lateral wall activation delay, measured by invasive electro-anatomical mapping, than with QRSd or QRS morphology.45 In patients with bradycardia, QRSa has been studied and compared during RV septal, deep septal and left bundle branch area pacing.45 Unfortunately, it has never been studied and compared in patients with various types of RV pacing.
ECG imaging (ECGi) is a complex, non-invasive imaging tool based on body surface potential mapping (BSPM). It reconstructs electro-anatomical epicardial activation from a combination of approximately 240 surface electrodes and computed tomography (CT) acquired heart-torso geometry (Figure 1). It creates over 2,500 epicardial unipolar electrocardiograms.
From these, a variety of interventricular as well as LV or RV dyssynchrony parameters can be calculated.46 These include: ventricular electrical uncoupling (VEU), which is the difference between mean LV and RV activation times and is thus considered to be an interventricular dyssynchrony parameter; LV total activation time (LVTAT); and the difference between the maximum and minimum activation times – the total activation time (TAT).
ECGi has been used primarily in patients with heart failure and various types of ventricular conduction defects or RV apical pacing.47 These studies show that the method provides detailed information about ventricular depolarisation patterns and predicts the response of these patients to biventricular resynchronisation therapy.48,49 No study has used ECGi to show the differences between various types of pacing in bradycardia patients or PICM prediction.
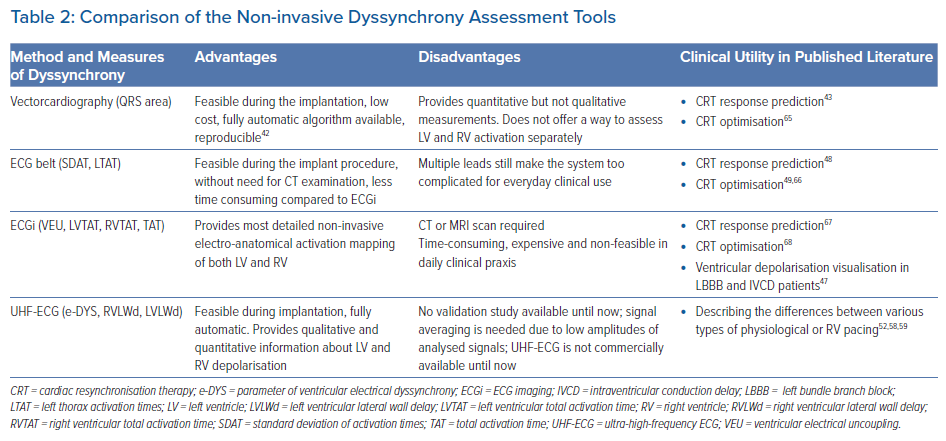
The ECG belt (Medtronic) is a simplified BSPM system consisting of 40 body surface electrodes, which do not require a CT or MRI scan for dyssynchrony assessment. The data are processed offline and generate colour-coded isochronal maps from the anterior and posterior chest view (Figure 1). The most often used dyssynchrony parameters derived from the ECG belt are the standard deviation of activation times (SDAT) and left thorax activation times (LTAT). These parameters have shown to be predictive of CRT response and useful for optimising CRT therapy.48,49
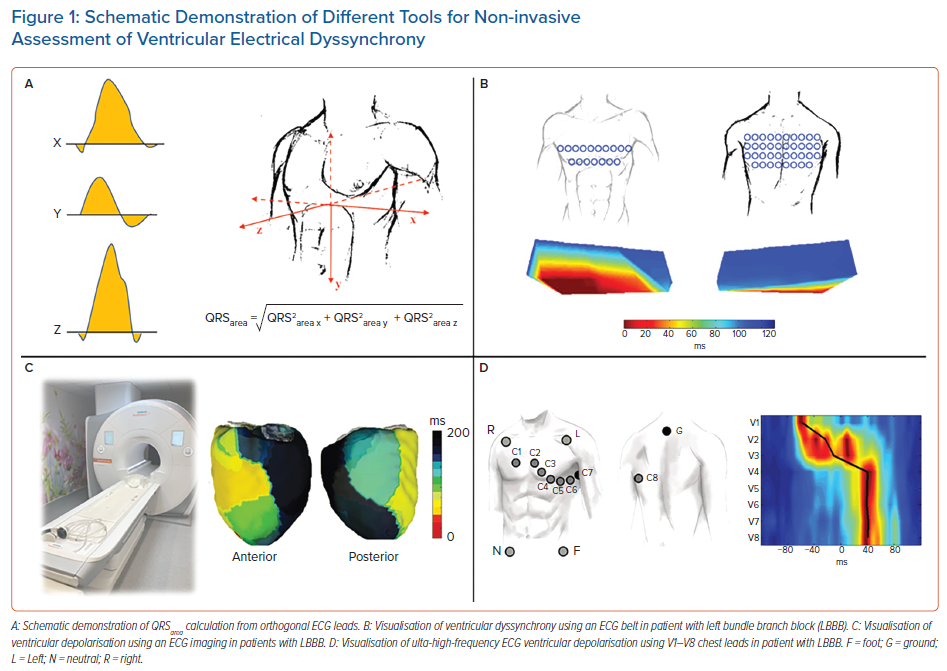
Compared to ECGi, the method is less expensive, less time consuming and easier to operate, which enables its use during implant procedures. However, the need for additional chest leads and the complexity of visualisation of ventricular depolarisation patterns make it less applicable in standard clinical care.
Ventricular activation patterns and dyssynchrony parameters can also be measured using ultra-high-frequency ECG (UHF-ECG), which is currently available for non-commercial, research purposes in a limited number of clinical centres. UHF-ECG displays the sequence of ventricular activation using an analysis of the ultra-high frequency components of ventricular myocyte action potentials in perimyocardial tissue.50,51
The ventricular activation sequence under standard chest leads (V1–V6 or V1–V8 configuration) is displayed in depolarisation maps, usually in 1–3 minutes, making the method suitable for clinical practice. The broad-band QRS complex is constructed as the average of the 16 normalised median amplitude envelopes of the 16 frequency bands (150–1000 Hz) and displayed as a coloured map for chest leads.
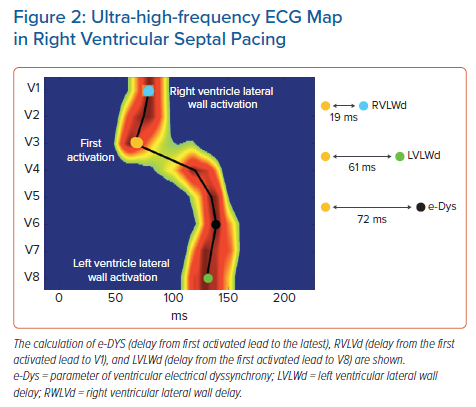
Local activation times are calculated as the centre of mass of the UHF-QRS above the 50% threshold of the baseline-to-peak amplitude for each chest lead. The parameter of ventricular electrical dyssynchrony – e-DYS – is calculated using the time difference between the first and last activated centre of mass.
Additional and more specific parameters, such as RV or LV lateral wall activation delay (RVLWd or LVLWd) as a distance from the first activated centre of mass to V1 and V8, respectively, can be calculated in milliseconds (ms) (Figure 2). A comparison of advantages, disadvantages, and possible clinical utility of non-invasive dyssynchrony assessment tools is summarised in Table 2.
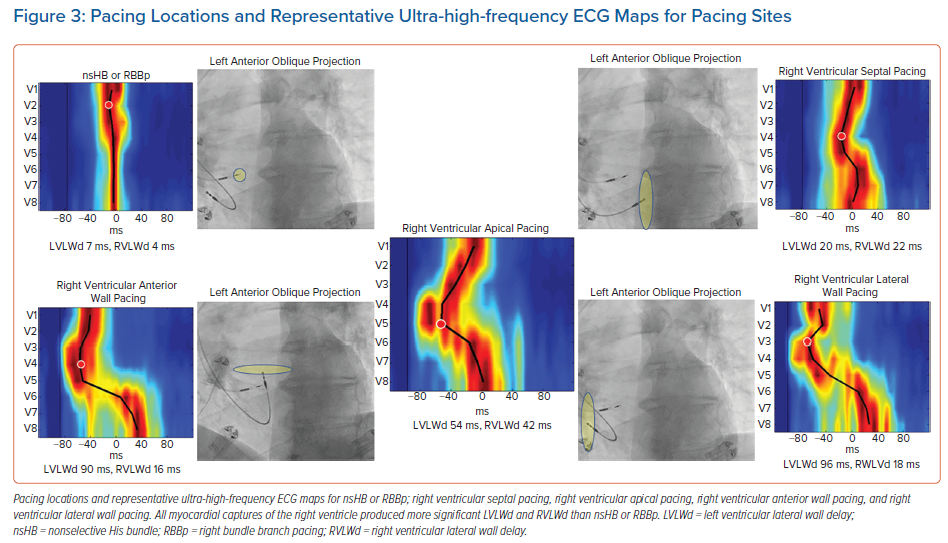
Recently, using a UHF-ECG, Curila et al. showed differences in ventricular activation patterns during pacing.52 In their study, they paced various RV locations during the implant procedure in patients with bradycardia. They showed that significant differences in RV and LV activation delays were present during pacing from the basal septum with myocardial and His bundle or right bundle branch engagement (nonselective His bundle or right bundle branch pacing), the pacing of the RV septum with pure myocardial capture, the pacing of the RV apex, and pacing of the RV anterior or RV lateral wall (Figure 3). The shortest LVLWd was observed during nonselective His bundle or right bundle branch pacing, while the longest LVLWd was observed during RV anterior and lateral wall pacing. A slight difference in LVLWd between RV septal and apical pacing was observed, although the latter caused a much longer QRSd.
Curila et al. also showed that significant differences exist between various pacing locations on the RV septum.52 Pacing the RV inflow tract caused a significantly shorter LVLWd than pacing septal myocytes in the RV outflow tract, during which LVLWd values were very similar to values seen during RV apical pacing. RV apical capture was the only studied capture type that caused significant RV activation delays. Variations in the RV and LV activation delay could be explained by differences between pacing locations and the character of the electrical wave-front propagation in both ventricles.
When the velocity of depolarisation wave-front propagation was measured in the leads placed above the LV lateral wall, it was found to be similar during RV apical, anterior and lateral wall pacing, and all were significantly longer compared to RV septal pacing. This is likely to be a result of different types of electrical wave-front propagation. During RV septal pacing, the LV Purkinje system is used for activation; however, during RV apical, anterior, and lateral wall pacing, slow myocardial cell-to-cell propagation plays a more significant role.
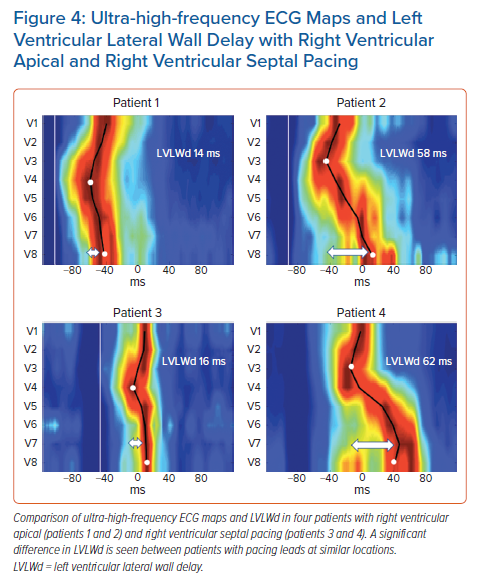
Although the averaged values showed significant differences between RV pacing sites, a closer review of the data revealed significant individual variability between the patients included in the study (data not published). There were patients with minimal LV and RV lateral wall delays during RV apical or RV septal pacing, but there were others in whom pacing the same locations resulted in much greater ventricular dyssynchrony (Figure 4).
In summary, UHF-ECG can visualise ventricular depolarisation patterns in various types of ventricular pacing during the implant procedure. Significant differences have been found between the pacing locations studied and individual patients using the same pacing locations. A multicentre clinical trial to determine if UHF-ECG dyssynchrony can serve as an additional tool for predicting patients with the highest risk of PICM has been started (NCT04908033). Unfortunately, the availability of UHF-ECG is limited. Until now, the hardware has been available only in nine centres and shortly it is going to be installed in another five centres.
Possible Prevention and Treatment of Pacing-induced Cardiomyopathy
Initially, PICM development was thought to result from RV apical pacing, which produced wide QRS complexes. It was hypothesised that narrowing the paced QRS duration during RV septal pacing would reduce PICM development. Unfortunately, no clinical trial comparing RV septal to apical pacing showed any clinical benefit of RV septal pacing.53,54 Additionally, no benefits in mortality or HF hospitalisations were observed in trials comparing biventricular pacing with RV pacing in patients with bradycardia.55,56
However, some of these studies had important shortcomings, which limited the potential benefit of reduced ventricular dyssynchrony during RV septal over RV apical pacing. RV septal lead placement was based on unreliable ECG or X-ray criteria, which led to incorrect lead fixations towards the anterior wall in a substantial percentage of patients, i.e. the pacing location, which, based on UHF-ECG data, can lead to more delayed LV lateral wall depolarisation than RV apical pacing.56,57
Recently, His-Purkinje conductive system pacing techniques were introduced. These include His bundle pacing (HBP), left bundle branch pacing (LBBP) and left ventricular septal pacing (LVSP). As shown recently, these techniques better preserve physiological ventricular activation than RV pacing.52,58,59 This favourable effect on ventricular activation during HBP was shown to reduce HF hospitalisations in patients requiring more than 20% ventricular pacing compared to RV apical or septal pacing.60
However, as shown subsequently, HBP has some limitations, such as higher pacing thresholds, which can lead to premature battery depletion, lower sensing values and lower success rates in patients with bundle branch blocks; these limitations have restricted its use in all patients.61,62
Moreover, in some studies, the risk of reintervention on pacing lead repositioning was unacceptably high.63 For that reason, more distal pacing lead placement (i.e., LBBP or LVSP) is now preferred by many specialists. Although these methods are less physiological than HBP, a recent multicentre, observational study showed they reduce the incidence of death and HF hospitalisations compared to RV apical or septal pacing.64
Whether these approaches will lead to a better clinical outcome than RV pacing needs to be demonstrated in prospective, randomised clinical trials. Even if these promising pacing methods prove effective, they are still more complex and require dedicated implant tools and advanced equipment in the operating room.
For that reason, at least for now, these methods are probably best suited for patients at the highest risk of pacing-induced cardiomyopathy.
Conclusion
Declining LVEF and the development of HF, as the main signs of pacing-induced cardiomyopathy, are not uncommon complications of permanent RV pacing.
These complications occur because of non-physiological ventricular activation, with resultant asynchronous ventricular contractions, which are detectable soon after the start of RV pacing.
Several methods based on the processing of signals generated by ventricular depolarisation or echocardiography can be used to assess ventricular dyssynchrony. While these methods provide the electrophysiologist with exact information about the resultant pattern of ventricular depolarisation associated with a specific pacing location, they are complex, time consuming and cannot be readily performed during standard implant procedures.
However, this information can be obtained using UHF-ECG, which can be used to visualise ventricular depolarisation patterns. This method analyses high-frequency ECG signals in a 12- or 14-lead ECG. It uses standard chest leads, and information about ventricular activation is available in less than 3 minutes. UHF-ECG is a promising method for real-time feedback during implant procedures since it visualises and quantifies the activation delay of specific ventricular segments. This additional information helps the electrophysiologist avoid pacing locations that produce dyssynchronous ventricular activation.
Clinical Perspective
- Dyssynchronous ventricular activation is one of the most important factors responsible for right ventricular pacing having a deleterious effect, and occurs in a subset of patients with pacemakers.
- Identifying patients with ventricular dyssynchrony during pacing would allow the most appropriate pacing method to be selected.
- Ventricular dyssynchrony can be measured using echocardiography or complex methods of ventricular depolarisation visualisation, but these are not well suited for use during standard implant procedures.
- Ultra-high-frequency ECGs can visualise the sequence of ventricular activation and identify patients with dyssynchronous ventricular depolarisation during pacing.
- If ultra-high-frequency ECG demonstrates an ability to predict patients at risk of decreased left ventricular ejection fraction due to pacing, then it may help to individualise treatment in patients with bradycardia.