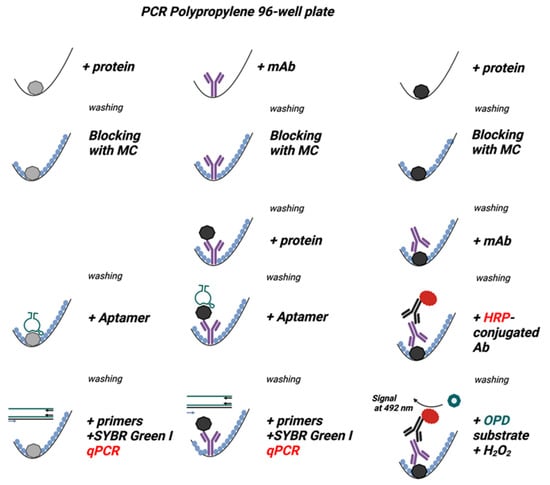
Simplified PCR-Based Quantification of Proteins with DNA Aptamers and Methylcellulose as a Blocking Agent
Abstract
:1. Introduction
2. Results
2.1. Binding of DNA Aptamers to Polypropylene Wells and Search for Agents Blocking This Binding
2.2. The ssDNA and Double-Stranded (dd)DNA Bind to Polypropylene Wells Comparably
2.3. Monoclonal Antibody (mAb) Binds to Polypropylene PCR Wells, and Methylcellulose Is a Good Blocker of Its Binding
2.4. Methylcellulose Efficiently Blocks the Nonspecific Binding of DNA Aptamers Used to Detect Target Proteins in Polypropylene Wells for PCR
2.5. Aptamer-Based iPCR (A-iPCR) with Methylcellulose as a Blocking Agent Is a Sensitive One-Well Assay for Detecting Low Concentrations of an Analyte
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Isolation of the Aptamer 44/70, Specific for Taq DNA Polymerase
4.3. Nonspecific Binding of Aptamers to the Wells of Polypropylene PCR Plates
4.4. Quantification of Proteins Directly Immobilized in the Polypropylene PCR Wells
4.5. A-iPCR
4.6. ELISA
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Berezovski, M.V.; Musheev, M.U.; Drabovich, A.P.; Jitkova, J.V.; Krylov, S.N. Non-SELEX: Selection of aptamers without intermediate amplification of candidate oligonucleotides. Nat. Protoc. 2006, 1, 1359–1369. [Google Scholar] [CrossRef]
- Nitsche, A.; Kurth, A.; Dunkhorst, A.; Panke, O.; Sielaff, H.; Junge, W.; Muth, D.; Scheller, F.; Stocklein, W.; Dahmen, C.; et al. One-step selection of Vaccinia virus-binding DNA aptamers by MonoLEX. BMC Biotechnol. 2007, 7, 48. [Google Scholar] [CrossRef]
- Peng, L.; Stephens, B.J.; Bonin, K.; Cubicciotti, R.; Guthold, M. A combined atomic force/fluorescence microscopy technique to select aptamers in a single cycle from a small pool of random oligonucleotides. Microsc. Res. Tech. 2007, 70, 372–381. [Google Scholar] [CrossRef]
- Fan, M.; McBurnett, S.R.; Andrews, C.J.; Allman, A.M.; Bruno, J.G.; Kiel, J.L. Aptamer selection express: A novel method for rapid single-step selection and sensing of aptamers. J. Biomol. Tech. 2008, 19, 311–319. [Google Scholar]
- Hmila, I.; Wongphatcharachai, M.; Laamiri, N.; Aouini, R.; Marnissi, B.; Arbi, M.; Sreevatsan, S.; Ghram, A. A novel method for detection of H9N2 influenza viruses by an aptamer-real time-PCR. J. Virol. Methods 2017, 243, 83–91. [Google Scholar] [CrossRef]
- Mairal, T.; Ozalp, V.C.; Lozano, S.P.; Mir, M.; Katakis, I.; O’Sullivan, C.K. Aptamers: Molecular tools for analytical applications. Anal. Bioanal. Chem. 2008, 390, 989–1007. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Lapa, S.A.; Chudinov, A.V.; Timofeev, E.N. The toolbox for modified aptamers. Mol. Biotechnol. 2016, 58, 79–92. [Google Scholar] [CrossRef]
- Xiang, W.; Lv, Q.; Shi, H.; Xie, B.; Gao, L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta 2020, 214, 120716. [Google Scholar] [CrossRef]
- Lao, Y.H.; Chiang, H.Y.; Yang, D.K.; Peck, K.; Chen, L.C. Selection of aptamers targeting the sialic acid receptor of hemagglutinin by epitope-specific SELEX. Chem. Commun. (Camb.) 2014, 50, 8719–8722. [Google Scholar] [CrossRef]
- Sedighian, H.; Halabian, R.; Amani, J.; Heiat, M.; Amin, M.; Fooladi, A.A.I. Staggered Target SELEX, a novel approach to isolate non-cross-reactive aptamer for detection of SEA by apta-qPCR. J. Biotechnol. 2018, 286, 45–55. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, H.; Zhou, J.; Ma, Q.; Yan, W.; Zhao, L.; Wu, S.; Chen, H. Aptamer-targeting of Aleutian mink disease virus (AMDV) can be an effective strategy to inhibit virus replication. Sci. Rep. 2021, 11, 4649. [Google Scholar] [CrossRef]
- Sano, T.; Smith, C.L.; Cantor, C.R. Immuno-PCR: Very sensitive antigen detection by means of specific antibody-DNA conjugates. Science 1992, 258, 120–122. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Adler, M.; Wacker, R. Immuno-PCR: High sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol. 2005, 23, 208–216. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Adler, M.; Wacker, R. Detecting antigens by quantitative immuno-PCR. Nat. Protoc. 2007, 2, 1918–1930. [Google Scholar] [CrossRef]
- Chang, L.; Li, J.; Wang, L. Immuno-PCR: An ultrasensitive immunoassay for biomolecular detection. Anal. Chim. Acta 2016, 910, 12–24. [Google Scholar] [CrossRef]
- Ruzicka, V.; Marz, W.; Russ, A.; Gross, W. Immuno-PCR with a commercially available avidin system. Science 1993, 260, 698–699. [Google Scholar] [CrossRef]
- Zhou, H.; Fisher, R.J.; Papas, T.S. Universal immuno-PCR for ultra-sensitive target protein detection. Nucleic Acids Res. 1993, 21, 6038–6039. [Google Scholar] [CrossRef]
- Zhang, H.T.; Kacharmina, J.E.; Miyashiro, K.; Greene, M.I.; Eberwine, J. Protein quantification from complex protein mixtures using a proteomics methodology with single-cell resolution. Proc. Natl. Acad. Sci. USA 2001, 98, 5497–5502. [Google Scholar] [CrossRef]
- Potuckova, L.; Franko, F.; Bambouskova, M.; Draber, P. Rapid and sensitive detection of cytokines using functionalized gold nanoparticle-based immuno-PCR, comparison with immuno-PCR and ELISA. J. Immunol. Methods 2011, 371, 38–47. [Google Scholar] [CrossRef]
- Stegurova, L.; Draberova, E.; Bartos, A.; Draber, P.; Ripova, D.; Draber, P. Gold nanoparticle-based immuno-PCR for detection of tau protein in cerebrospinal fluid. J. Immunol. Methods 2014, 406, 137–142. [Google Scholar] [CrossRef]
- Potuckova, L.; Draberova, L.; Halova, I.; Paulenda, T.; Draber, P. Positive and negative regulatory roles of C-terminal Src kinase (CSK) in FcεRI-mediated mast cell activation, independent of the transmembrane adaptor PAG/CSK-binding protein. Front. Immunol. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Vogt, R.F., Jr.; Phillips, D.L.; Henderson, L.O.; Whitfield, W.; Spierto, F.W. Quantitative differences among various proteins as blocking agents for ELISA microtiter plates. J. Immunol. Methods 1987, 101, 43–50. [Google Scholar] [CrossRef]
- Xiao, Y.; Isaacs, S.N. Enzyme-linked immunosorbent assay (ELISA) and blocking with bovine serum albumin (BSA)--not all BSAs are alike. J. Immunol. Methods 2012, 384, 148–151. [Google Scholar] [CrossRef]
- Rebeski, D.E.; Winger, E.M.; Shin, Y.K.; Lelenta, M.; Robinson, M.M.; Varecka, R.; Crowther, J.R. Identification of unacceptable background caused by nonspecific protein adsorption to the plastic surface of 96-well immunoassay plates using a standardized enzyme-linked immunosorbent assay procedure. J. Immunol. Methods 1999, 226, 85–92. [Google Scholar] [CrossRef]
- Craig, W.Y.; Poulin, S.E.; Collins, M.F.; Ledue, T.B.; Ritchie, R.F. Background staining in immunoblot assays. Reduction of signal caused by cross-reactivity with blocking agents. J. Immunol. Methods 1993, 158, 67–76. [Google Scholar] [CrossRef]
- Craig, W.Y.; Poulin, S.E.; Nelson, C.P.; Ritchie, R.F. ELISA of IgG antibody to oxidized low-density lipoprotein: Effects of blocking buffer and method of data expression. Clin. Chem. 1994, 40, 882–888. [Google Scholar] [CrossRef]
- Redcenko, O.; Draberova, L.; Draber, P. Carboxymethylcellulose enhances the production of single-stranded DNA aptamers generated by asymmetric PCR. Anal. Biochem. 2020, 589, 113502. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Chevillard, C.; Axelos, M.A.V. Phase separation of aqueous solution of methylcellulose. J. Colloid Polym. Sci. 1997, 275, 537–545. [Google Scholar] [CrossRef]
- Ahirwar, R.; Bariar, S.; Balakrishnan, A.; Nahar, P. BSA blocking in enzyme-linked immunosorbent assays is a non-mandatory step: A perspective study on mechanism of BSA blocking in common ELISA protocols. RSC Adv. 2015, 5, 100077–100083. [Google Scholar] [CrossRef]
- Belotserkovskii, B.P.; Johnston, B.H. Polypropylene tube surfaces may induce denaturation and multimerization of DNA. Science 1996, 271, 222–223. [Google Scholar] [CrossRef]
- Gaillard, C.; Strauss, F. Avoiding adsorption of DNA to polypropylene tubes and denaturation of short DNA fragments. Tech. Tips Online 1998, 3, 63–65. [Google Scholar] [CrossRef]
- Gaillard, C.; Flavin, M.; Woisard, A.; Strauss, F. Association of double-stranded DNA fragments into multistranded DNA structures. Biopolymers 1999, 50, 679–689. [Google Scholar] [CrossRef]
- Feng, B.; Sosa, R.P.; Martensson, A.K.F.; Jiang, K.; Tong, A.; Dorfman, K.D.; Takahashi, M.; Lincoln, P.; Bustamante, C.J.; Westerlund, F.; et al. Hydrophobic catalysis and a potential biological role of DNA unstacking induced by environment effects. Proc. Natl. Acad. Sci. USA 2019, 116, 17169–17174. [Google Scholar] [CrossRef]
- Barone, G.; Fonseca, G.C.; Bickelhaupt, F.M. B-DNA structure and stability as function of nucleic acid composition: Dispersion-corrected DFT study of dinucleoside monophosphate single and double strands. ChemistryOpen 2013, 2, 186–193. [Google Scholar] [CrossRef]
- Bodvik, R.; Karlson, L.; Edwards, K.; Eriksson, J.; Thormann, E.; Claesson, P.M. Aggregation of modified celluloses in aqueous solution: Transition from methylcellulose to hydroxypropylmethylcellulose solution properties induced by a low-molecular-weight oxyethylene additive. Langmuir 2012, 28, 13562–13569. [Google Scholar] [CrossRef]
- Mohammad, K.; Esen, A. A blocking agent and a blocking step are not needed in ELISA, immunostaining dot-blots and western blots. J. Immunol. Methods 1989, 117, 141–145. [Google Scholar] [CrossRef]
- Esser, P. Detergent in polystyrene ELISA. Nunc. Bull. 1990, 8, 1–6. [Google Scholar]
- Esser, P. Blocking agent and detergent in ELISA. Nunc. Bull. 1991, 9, 1–4. [Google Scholar]
- Schmitz, A.; Weber, A.; Bayin, M.; Breuers, S.; Fieberg, V.; Famulok, M.; Mayer, G. A SARS-CoV-2 spike binding DNA aptamer that inhibits pseudovirus infection by an RBD-independent mechanism. Angew. Chem. Int. Ed. Engl. 2021, 60, 10279–10285. [Google Scholar] [CrossRef]
- Shaik, G.M.; Dráberová, L.; Dráber, P.; Boubelík, M.; Dráber, P. Tetraalkylammonium derivatives as real-time PCR enhancers and stabilizers of the qPCR mixtures containing SYBR Green I. Nucleic Acids Res. 2008, 36, e93. [Google Scholar] [CrossRef]
- Cho, S.J.; Woo, H.M.; Kim, K.S.; Oh, J.W.; Jeong, Y.J. Novel system for detecting SARS coronavirus nucleocapsid protein using an ssDNA aptamer. J. Biosci. Bioeng. 2011, 112, 535–540. [Google Scholar] [CrossRef]
- Bovaird, J.H.; Ngo, T.T.; Lenhoff, H.M. Optimizing the o-phenylenediamine assay for horseradish peroxidase: Effects of phosphate and pH, substrate and enzyme concentrations, and stopping reagents. Clin. Chem. 1982, 28, 2423–2426. [Google Scholar] [CrossRef]
- Fornera, S.; Walde, P. Spectrophotometric quantification of horseradish peroxidase with o-phenylenediamine. Anal. Biochem. 2010, 407, 293–295. [Google Scholar] [CrossRef]






| SP6 aptamer * | GGGAGAGGAGGGAGATAGATATCAACCCATGGTAGGTATTGCTTGGTAGGGATAGTGGGCTTGATGTTTCGTGGATGCCACAGGAC |
| F primer | GGGAGAGGAGGGAGATAGATATCAA |
| R primer | GTCCTGTGGCATCCACGAAA |
| Complementary to SP6 | GTC CTG TGG CAT CCA CGA AAC ATC AAG CCC ACT ATC CCT ACC AAG CAA TAC CTA CCA TGG GTT GAT ATC TAT CTC CCT CCT CTC CC |
| 44/70 aptamer ** | CCTTGAACCTGTGCCATTTGCTAATTGAGACTATTATGGGCTTTTTAGTCGAACAGTAGGAAGATGGAGG |
| F primer | CCTTGAACCTGTGCCATTTG |
| R primer | CCTCCATCTTCCTACTGTTC |
| NC aptamer *** | GCAATGGTACGGTACTTCCGGATGCGGAAACTGGCTAATTGGTGAGGCTGGGGCGGTCGTGCAGCAAAAGTGCACGCTACTTTGCTAA |
| F primer | GCAATGGTACGGTACTTCC |
| R primer | TTAGCAAAGTAGCGTGCACTTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redcenko, O.; Tumova, M.; Draber, P. Simplified PCR-Based Quantification of Proteins with DNA Aptamers and Methylcellulose as a Blocking Agent. Int. J. Mol. Sci. 2024, 25, 347. https://doi.org/10.3390/ijms25010347
Redcenko O, Tumova M, Draber P. Simplified PCR-Based Quantification of Proteins with DNA Aptamers and Methylcellulose as a Blocking Agent. International Journal of Molecular Sciences. 2024; 25(1):347. https://doi.org/10.3390/ijms25010347
Chicago/Turabian StyleRedcenko, Oleksij, Magda Tumova, and Petr Draber. 2024. "Simplified PCR-Based Quantification of Proteins with DNA Aptamers and Methylcellulose as a Blocking Agent" International Journal of Molecular Sciences 25, no. 1: 347. https://doi.org/10.3390/ijms25010347