Abstract
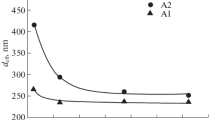
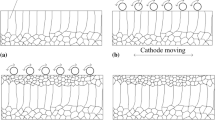
The high surface roughness of thick and dense alumina deposits prepared by long-term electrophoretic deposition (EPD) caused by surface inhomogeneities could be eliminated by changing the suspension composition or EPD parameters. This work is aimed at the influence of the suspension composition, more precisely on the increase of electrical conductivity through indifferent electrolyte (lithium chloride) addition and its effect on the surface roughness. At first, the electrical conductivity was adjusted by the various amounts of the stabilisers of mono-, di- and trichloroacetic acid in the range of 0.85–21.25 wt%. It was demonstrated that deposits prepared from these suspensions were several millimetres thick, dense with a relatively low surface roughness (Ra ≈ 10 μm) only when the electrical conductivity was higher than 4 μS/cm. If a portion of the stabiliser was replaced with indifferent electrolyte, it resulted in the significantly smoother surface with a roughness Ra ≈ 2 μm preserving all other benefits. Suggested optimization represents a useful novel approach for the preparation of dense, thick and near net-shaped alumina deposits with low surface roughness via EPD.





Similar content being viewed by others
References
Sarkar, P., Nicholson, P.S.: Electrophoretic deposition (EPD): mechanisms, kinetics, and application to ceramics. J. Am. Ceram. Soc. 79(8), 1987–2002 (1996). https://doi.org/10.1111/j.1151-2916.1996.tb08929.x
Besra, L., Uchikoshi, T., Suzuki, T.S., Sakka, Y.: Application of constant current pulse to suppress bubble incorporation and control deposit morphology during aqueous electrophoretic deposition (EPD). J. Eur. Ceram. Soc. 29(10), 1837–1845 (2009). https://doi.org/10.1016/j.jeurceramsoc.2008.07.031
Tahmasbi Rad, A., Solati-Hashjin, M., Osman, N.A.A., Faghihi, S.: Improved bio-physical performance of hydroxyapatite coatings obtained by electrophoretic deposition at dynamic voltage. Ceram. Int. 40(8, Part B), 12681–12691 (2014). https://doi.org/10.1016/j.ceramint.2014.04.116
Besra, L., Liu, M.: A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 52(1), 1–61 (2007). https://doi.org/10.1016/j.pmatsci.2006.07.001
Diba, M., Fam, D.W.H., Boccaccini, A.R., Shaffer, M.S.P.: Electrophoretic deposition of graphene-related materials: a review of the fundamentals. Prog. Mater. Sci. 82, 83–117 (2016). https://doi.org/10.1016/j.pmatsci.2016.03.002
Ferrari, B., Moreno, R.: Electrophoretic deposition of aqueous alumina slips. J. Eur. Ceram. Soc. 17(4), 549–556 (1997). https://doi.org/10.1016/S0955-2219(96)00113-6
Milani, M., Zahraee, S.M., Mirkazemi, S.M.: Influence of electrophoretic deposition parameters on pore size distribution of doped nano alumina plates. Ceramics-Silikáty. 60(4), 299–307 (2016). https://doi.org/10.13168/cs.2016.0045
Hasanpoor, M., Aliofkhazraei, M., Delavari, H.,.H.: In-situ study of mass and current density for electrophoretic deposition of zinc oxide nanoparticles. Ceram. Int. 42(6), 6906–6913 (2016). https://doi.org/10.1016/j.ceramint.2016.01.076
Javidi, M., Javadpour, S., Bahrololoom, M.E., Ma, J.: Electrophoretic deposition of natural hydroxyapatite on medical grade 316L stainless steel. Mater. Sci. Eng. C. 28(8), 1509–1515 (2008). https://doi.org/10.1016/j.msec.2008.04.003
Meng, X., Kwon, T.Y., Yang, Y., Ong Joo, L., Kim, K.H.: Effects of applied voltages on hydroxyapatite coating of titanium by electrophoretic deposition. J Biomed Mater Res B Appl Biomater. 78B(2), 373–377 (2006). https://doi.org/10.1002/jbm.b.30497
Hadraba, H., Chlup, Z., Drdlik, D., Cihlar, J.: Micro-fibres containing composites prepared by EPD. J. Eur. Ceram. Soc. 36(2), 365–371 (2016). https://doi.org/10.1016/j.jeurceramsoc.2015.07.031
Maca, K., Pouchly, V., Drdlik, D., Hadraba, H., Chlup, Z.: Dilatometric study of anisotropic sintering of alumina/zirconia laminates with controlled fracture behaviour. J. Eur. Ceram. Soc. 37(14), 4287–4295 (2017). https://doi.org/10.1016/j.jeurceramsoc.2017.04.030
Hung, C.-W., Chang, C.-H., Chen, W.-C., Chen, C.-C., Chen, H.-I., Tsai, Y.-T., Tsai, J.-H., Liu, W.-C.: A Pt/AlGaN/GaN heterostructure field-effect transistor (HFET) prepared by an electrophoretic deposition (EPD)-gate approach. Solid State Electron. 124, 5–9 (2016). https://doi.org/10.1016/j.sse.2016.06.011
Keller, F., Nirschl, H., Dörfler, W., Woldt, E.: Efficient numerical simulation and optimization in electrophoretic deposition processes. J. Eur. Ceram. Soc. 35(9), 2619–2630 (2015). https://doi.org/10.1016/j.jeurceramsoc.2015.02.031
Michaud, X., Shi, K., Zhitomirsky, I.: Electrophoretic deposition of LiFePO4 for Li-ion batteries. Mater. Lett. 241, 10–13 (2019). https://doi.org/10.1016/j.matlet.2019.01.032
Esfahani, S.L., Rouhani, S., Ranjbar, Z.: Optimization the electrophoretic deposition fabrication of graphene-based electrode to consider electro-optical applications. Surf. Interfaces. 9, 218–227 (2017). https://doi.org/10.1016/j.surfin.2017.10.001
Song, G., Xu, G., Quan, Y., Yuan, Q., Davies, P.A.: Uniform design for the optimization of Al2O3 nanofilms produced by electrophoretic deposition. Surf. Coat. Technol. 286, 268–278 (2016). https://doi.org/10.1016/j.surfcoat.2015.12.039
Drdlik, D., Moravek, T., Rahel, J., Stupavska, M., Cihlar, J., Drdlikova, K., Maca, K.: Electrophoretic deposition of plasma activated sub-micron alumina powder. Ceram. Int. 44(8), 9787–9793 (2018). https://doi.org/10.1016/j.ceramint.2018.02.215
Ervina, J., Ghaleb, Z.A., Hamdan, S., Mariatti, M.: Colloidal stability of water-based carbon nanotube suspensions in electrophoretic deposition process: effect of applied voltage and deposition time. Compos. A: Appl. Sci. Manuf. 117, 1–10 (2019). https://doi.org/10.1016/j.compositesa.2018.11.002
Ferrari, B., Moreno, R.: The conductivity of aqueous Al2O3 slips for electrophoretic deposition. Mater. Lett. 28(4), 353–355 (1996). https://doi.org/10.1016/0167-577X(96)00075-4
Lefrou, C., Fabry, P., Poignet, J.C.: Electrochemistry: the Basics, with Examples. Springer, Berlin Heidelberg (2012)
Cruz, R.C.D., Reinshagen, J., Oberacker, R., Segadães, A.M., Hoffmann, M.J.: Electrical conductivity and stability of concentrated aqueous alumina suspensions. J. Colloid Interface Sci. 286(2), 579–588 (2005). https://doi.org/10.1016/j.jcis.2005.02.025
Liu, X., Maki-Arvela, P., Aho, A., Vajglova, Z., Gun’ko, V.M., Heinmaa, I., Kumar, N., Eranen, K., Salmi, T., Murzin, D.Y.: Zeta potential of beta zeolites: influence of structure, acidity, pH, temperature and concentration. Molecules (Basel, Switzerland). 23(4), (2018). https://doi.org/10.3390/molecules23040946
Krüger, H.G., Knote, A., Schindler, U., Kern, H., Boccaccini, A.R.: Composite ceramic-metal coatings by means of combined electrophoretic deposition and galvanic methods. J. Mater. Sci. 39(3), 839–844 (2004). https://doi.org/10.1023/B:JMSC.0000012912.96350.d2
Cihlar, J., Drdlik, D., Cihlarova, Z., Hadraba, H.: Effect of acids and bases on electrophoretic deposition of alumina and zirconia particles in 2-propanol. J. Eur. Ceram. Soc. 33(10), 1885–1892 (2013). https://doi.org/10.1016/j.jeurceramsoc.2013.02.017
Safarík, L., Stránský, Z.: Titrimetric Analysis in Organic Solvents. Elsevier, Amsterdam (1986)
Izutsu, K.: Electrochemistry in Nonaqueous Solutions. Wiley, (2009)
Ji, C., Lan, W., Xiao, P.: Fabrication of yttria-stabilized zirconia coatings using electrophoretic deposition: packing mechanism during deposition. J. Am. Ceram. Soc. 91(4), 1102–1109 (2008). https://doi.org/10.1111/j.1551-2916.2008.02261.x
Guo, F., Shapiro, I.P., Xiao, P.: Effect of HCl on electrophoretic deposition of yttria stabilized zirconia particles in organic solvents. J. Eur. Ceram. Soc. 31(14), 2505–2511 (2011). https://doi.org/10.1016/j.jeurceramsoc.2011.02.027
Anné, G., Vanmeensel, K., Neirinck, B., Van der Biest, O., Vleugels, J.: Ketone-amine based suspensions for electrophoretic deposition of Al2O3 and ZrO2. J. Eur. Ceram. Soc. 26(16), 3531–3537 (2006). https://doi.org/10.1016/j.jeurceramsoc.2006.01.019
Hirata, Y., Nishimoto, A., Ishihara, Y.: Forming of alumina powder by electrophoretic deposition. J. Ceram. Soc. Jpn. International ed. 99(2), 105–109 (1991)
Kreethawate, L., Larpkiattaworn, S., Jiemsirilers, S., Besra, L., Uchikoshi, T.: Application of electrophoretic deposition for inner surface coating of porous ceramic tubes. Surf. Coat. Technol. 205(7), 1922–1928 (2010). https://doi.org/10.1016/j.surfcoat.2010.08.069
Basu, R.N., Randall, C.A., Mayo, M.J.: Fabrication of dense zirconia electrolyte films for tubular solid oxide fuel cells by electrophoretic deposition. J. Am. Ceram. Soc. 84(1), 33–40 (2001). https://doi.org/10.1111/j.1151-2916.2001.tb00604.x
Hamaker, H.C., Verwey, E.J.W.: The role of the forces between the particles in electrodeposition and other phenomena. Trans. Faraday Soc. 36, 180–185 (1940)
Zhitomirsky, I., Gal-Or, L.: Electrophoretic deposition of hydroxyapatite. J. Mater. Sci. Mater. Med. 8(4), 213–219 (1997)
Trau, M., Seville, D.A., Aksay, I.A.: Field-induced layering of colloidal crystals. Science. 272(5262), 706–709 (1996)
Meyers, D.: Surfaces, Interfaces, and Colloids, vol. 2. Wiley-VCH, New York (1999)
Funding
The authors acknowledge the support of the Grant Agency of the Czech Republic under grant no. 17-08153S. This research was also carried out under the project CEITEC 2020 (LQ1601) with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme II.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drdlik, D., Chlup, Z., Hadraba, H. et al. Surface roughness improvement of near net shaped alumina by EPD. J Aust Ceram Soc 56, 721–727 (2020). https://doi.org/10.1007/s41779-019-00390-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-019-00390-y