Abstract
The Mashona mole-rat, Fukomys darlingi (Thomas, 1895), is a little studied social African mole-rat (Bathyergidae) from south-astern Africa. Here, we present an integrative study characterizing the genetic diversity of populations assigned to F. darlingi with special focus on animals from Nsanje, southern Malawi. These mole-rats show pronounced differences in body mass and general appearance compared to nominate F. darlingi from Zimbabwe and Mozambique, but their taxonomic status has so far remained unclear. A genetic analysis encompassing all major lineages of the genus Fukomys suggests that this population indeed represents a deeply nested lineage within the F. darlingi clade. The karyotype of the Nsanje mole-rats also corresponds to that of the nominate form, being 2n = 54. While both nuclear and mitochondrial data agree about the assignment of the Nsanje mole-rats to F. darlingi, our analyses revealed substantial mitonuclear discordance for other branches within the Fukomys phylogenetic tree. Nsanje mole-rats are significantly larger than nominate F. darlingi and their ontogeny and reproduction closely resemble similar-sized congeneric species rather than the nominate population. The somatic growth of the Nsanje form is the slowest of all African mole-rats. The maximum life span of F. darlingi is at least 19 years. The observed differences between nominate F. darlingi and mole-rats from Nsanje may be attributed mainly to their different body mass. Our study highlights the advantages of an integrative approach for understanding the diversity of African mole-rats and emphasizes the great intraspecific variability that may be encountered in these underground-dwelling rodents.
Similar content being viewed by others
Introduction
The African mole-rats (Bathyergidae), a family of strictly subterranean rodents, have attracted great scientific attention due to many peculiar aspects of their biology. This group comprises six genera, Cryptomys (southern common mole-rats), Fukomys (northern common mole-rats), Heterocephalus (naked mole-rat), Georychus (Cape mole-rat), Bathyergus (dune mole-rats), and Heliophobius (silvery mole-rat), which predominately occur in the savannah habitats of sub-Saharan Africa. All of them have been subjected to multiple physiological, behavioural, and phylogenetic studies (reviewed in Begall et al., 2018; Bennett & Faulkes, 2000). For many reasons, however, the life histories, species diversity, and geographic distribution of many taxa are still little known. Considering general biology and life history characteristics, we only possess relatively complete information for a few species such as the Damaraland mole-rat ((Fukomys damarensis (Ogilby, 1838)) of the Kalahari sands, the Zambian Ansell’s mole-rat ((Fukomys anselli (Burda et al., 1999)), and the naked mole-rat (Heterocephalus glaber, Rüppel, 1842) from the Horn of Africa. For most other bathyergid taxa, such data is still lacking or largely anecdotal. Estimates of bathyergid species diversity are hindered by the fact that many taxa were described or established based on singular lines of evidence from either morphological studies (e.g., Gippoliti & Amori, 2011; Thomas, 1895), karyological differences (e.g., Aguilar, 1993; Burda et al., 1999), or single-gene phylogenies (e.g., Faulkes et al., 2011). Currently, integrative approaches to bathyergid taxonomy are lacking (but see Van Daele et al., 2013).
For a long time, common mole-rats, the most diverse and widespread bathyergid radiation, were all classified within the genus Cryptomys (Gray, 1864; Thomas, 1917; Wilson & Reeder, 2005). Molecular methods revealed that the morphologically rather uniform species, which were traditionally comprised by Cryptomys, form in fact two deeply diverging cryptic lineages, which are now differentiated at the generic level as Cryptomys and Fukomys (Faulkes et al., 2004; Ingram et al., 2004; Kock et al., 2006). Both Cryptomys and Fukomys are social and live in cooperatively breeding groups, typically structured around a breeding pair. However, while there is evidence for low male reproductive skew and the regular occurrence of genetic polyandry in Cryptomys, Fukomys is predominately monogamous (Bishop et al., 2004; Burland et al., 2002; Patzenhauerová et al., 2013). Furthermore, offspring dispersal is more delayed in most Fukomys species compared to Cryptomys, leading to a greater group size in the former (Begall et al., 2018).
Whereas Cryptomys is exclusively found in southern Africa, Fukomys ranges from the north of the Republic of South Africa to the northern, Sahelo-Sudanian, savannahs. Fukomys is the most speciose bathyergid genus, encompassing at least 14 (Kock et al., 2006; Monadjem et al., 2015) but most likely more species (Faulkes & Bennett, 2013; Mammal Diversity Database, 2022). Although there were notable efforts to elucidate Fukomys genetic diversity and taxonomy (Faulkes et al., 2017; Van Daele et al., 2004, 2007, 2013), many phylogenetic lineages within this genus remain severely understudied.
One such lineage of Fukomys occurs in the southernmost part of Malawi, in the Nsanje district, where it is especially common on the escarpment of Chididi (Ansell & Dowsett, 1988). Albeit relatively small, Malawi is a geomorphologically heterogenous country and its Fukomys populations show a disjunct distribution. Between the Nsanje district in the south and Kasungu near to the Viphya plateau in the north, there are no confirmed records of mole-rats (Ansell & Dowsett, 1988). The mole-rats living in northern Malawi have long been classified as Fukomys whytei (previously Cryptomys hottentotus whytei Thomas, 1897) and their distinctiveness has been repeatedly confirmed by genetic studies (Ingram et al., 2004; Van Daele et al., 2007; Visser et al., 2020). However, not all Fukomys in northern Malawi belong to that species. Surprisingly, a mole-rat population near Mzuzu was found to form a deeply divergent lineage within the genus (Faulkes et al., 2010). In one of three alternative phylogenies, these Mzuzu mole-rats shared a recent common ancestor with animals from Hanang in northern Tanzania (Faulkes et al., 2010) which were later on described as a distinct species, F. hanangensis (Faulkes et al., 2017). Without any proper justification, Visser et al. (2020) grouped the Mzuzu mole-rats into F. hanangensis, despite the long branch lengths and genetic distances separating the two lineages. All northern Malawian mole-rats live usually at altitudes above 1000 m a.s.l.
Common mole-rats in southern Malawi, in the Nsanje region, occurr at much lower altitudes along the Shire River. They are generally supposed to be isolated from both the mole-rats of northern Malawi and those ranging south of the Zambezi River in central Mozambique (Smithers & Tello, 1976). Their phylogenetic affinities have remained obscure. Apart from a potential relationship to F. whytei, there is also the possibility that the southern Malawi mole-rats are closely related or belong to the species Fukomys darlingi (Thomas, 1895), the Mashona mole-rat, which is distributed primarily in the highlands of eastern Zimbabwe and central Mozambique (Bennett & Faulkes, 2000). Finally, these animals could represent an undescribed species, given striking differences between southern Malawi mole-rats and both F. whytei from northern Malawi and F. darlingi from Zimbabwe. When compared to nominate F. darlingi in particular, southern Malawi mole-rats are notably larger and more homeothermic, i.e., exhibiting a more stable core body temperature across different ambient temperatures (see Bennett et al., 1994 and Zemanová et al., 2012 for comparison).
In this study, we use one mitochondrial and five nuclear gene sequences to determine the phylogenetic position of mole-rats from the Nsanje district of southern Malawi. Clarifying the taxonomic status of Nsanje mole-rats is important, because descendants of these animals breed in the labs of the University of South Bohemia and the University of Duisburg-Essen and are used for various behavioural and physiological studies (see Dvořáková et al., 2016; Wiedenová et al., 2018; Vejmělka et al., 2021; Caspar et al., 2021a). So far, we used the provisional name F. “Nsanje” for these animals or denoted them as closely related to F. darlingi based on very preliminary trees constructed from mitochondrial cytochrome b data. Finally, we provide information on reproductive and life history parameters of Nsanje mole-rats obtained from the two aforementioned labs and compare them to data on the nominate form of F. darlingi from Zimbabwe, as well as to other Fukomys species.
Methods
Study animals
Mole-rats were trapped in the vicinity of Nsanje town, southern Malawi (16° 55′S, 35° 15′E, altitude 53 m a.s.l.), in August 2005. Altogether, we obtained 16 individuals (5 males, 11 females) from different family groups (Supplementary Information 1). Ten individuals were exported to the Czech Republic, being the later founders of our captive breeding stock in the vivarium at the University of South Bohemia in České Budějovice. In July 2019, descendants of this lab lineage were transferred to the vivarium at the University of Duisburg-Essen.
Molecular data
Phylogenetic inference was based on sequences of the mitochondrial gene cytochrome b (CYTB) and five nuclear loci: recombination activating gene 1 (RAG1), intron 7 of the β-fibrinogen gene (FGB), intron 7 of 24-dehydrocholesterol reductase precursor (DHCR), intron 9 of smoothened homolog precursor (SMO), and intron 7 of transient receptor potential cation channel, subfamily V, member 4 (TRPV).
The CYTB tree included 59 sequences, 706–1140 base pairs (bp) long. Out of them, 27 sequences were newly obtained from fresh tissue samples (GenBank accessions: OQ559424–OQ559450) and 32 sequences, including three Cryptomys as outgroups, were taken from published data (Faulkes et al., 1997, 2004, 2010; 2017; Van Daele et al., 2007; Visser et al., 2018; Krásová et al., 2021). For molecular barcoding, 25 additional short (189 bp) CYTB sequences (GenBank accession: OQ559451–OQ559475) were obtained from specimens of the National History Museum of Zimbabwe (NMZB). The nuclear tree was based on 122 sequences from 25 individuals of Fukomys; (30 sequences published by Uhrová et al. (2022), 92 newly generated with GenBank accessions for RAG1: OQ442881–OQ442896, FGB: OQ442843–OQ442861, DHCR: OQ559483–OQ559501, TRPV: OQ559505–OQ559523, SMO: OQ442862–OQ442880) and as an outgroup we used 15 sequences of three individuals of Georychus capensis published by Uhrová et al. (2022). The nuclear data matrix was almost complete, only three ingroup sequences of RAG1 were missing. The length of the loci was 1066 bp (RAG1), 823 bp (FGB), 377 bp (DHCR), 433 bp (SMO), and 320 bp (TRPV).
DNA from fresh tissue samples was extracted using a commercial kit (DNeasy Blood & Tissue Kit, Qiagen) following the manufacturer’s protocol. Nucleotide sequences were amplified by polymerase chain reaction using the primers and protocols specified in Uhrová et al. (2022). The short CYTB sequences were obtained from museum material by amplicon sequencing on an Illumina MiSeq platform (Illumina, San Diego, CA, USA), using primers designed by Galan et al. (2012).
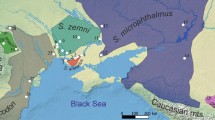
The CYTB-sequenced individuals cover almost the full geographic range of the genus Fukomys (Fig. 1), but special focus was put on the population from Nsanje (represented by two individuals) and mole-rats from adjacent regions, including several putative representatives of F. whytei and F. darlingi. The individuals with nuclear sequence data represented all main CYTB lineages in the genus. The complete list of the used material is provided in the Supplementary Information 2, which includes also GenBank accession numbers of the published sequences and geographical coordinates of the sampling sites, if available. Finally, Fig. 1 and Supplementary Information 2 also contain 30 additional records of F. darlingi for which GPS coordinates were obtained from Monadjem et al. (2015).
The geographic distribution of localities where mole-rats were sampled for this study. In the inset, full circles indicate localities represented by whole CYTB sequences, while empty symbols denote short CYTB sequences of museum samples. Red crosses show records of Fukomys darlingi individuals published in Monadjem et al. (2015). The symbol colours match those of the CYTB lineages illustrated in the trees shown in Figs. 4 and 5. Red outlines show the estimated range of F. darlingi according to the IUCN Red List (IUCN 2022). The samples from Ghana (Fukomys zechi: Atebubu: 7.75N, 1W) and Nigeria (Fukomys foxi: Panyam: 9.4104411N, 9.21362E) are not depicted. 1, Nsanje + Chididi; 2, Kasungu + Mzuzu; 3, Hanang
Phylogenetic methods
The CYTB tree was reconstructed by means of Bayesian inference as implemented in MrBayes 3.2.6 (Ronquist et al., 2012). The alignment was partitioned according to the codon position with GTR + F + G4 nucleotide substitution model (Tavaré, 1986; Yang, 1994) in each partition, as suggested by ModelFinder tool of IQ-TREE v1.6 (Kalyaanamoorthy et al., 2017; Nguyen et al., 2015), with AIC (Akaike, 1974) specified as the search criterion. The Markov chain Monte Carlo (MCMC) was run for 2 × 106 generations with sampling every 1000 generations and its convergence to posterior examined by comparison of four independent runs in Tracer 1.7 (Rambaut et al., 2018). From all four posterior samples, the first 20% of trees were discarded as burn-in and the remaining were pooled and outgroup-rooted, and the outgroups were removed. The sample of 6400 trees was represented by the maximum clade credibility (MCC) tree with the mean common ancestor node heights (Drummond & Bouckaert, 2015), which was calculated in R (R Core Team, 2022) using the function find_mcct available at https://github.com/onmikula/bpptools.
The CYTB lineages, representing putative species, were determined using the branch-cutting method (https://www.biorxiv.org/content/10.1101/419887v1.full) whose R implementation is available at https://github.com/onmikula/phyloeda. This method partitions a tree into lineages according to the importance of branches and assuming that speciation is such a unique process that deletion of any branch representing speciation would significantly change the structure of a given phylogeny. The partitioning was constrained by an assumption that individuals previously classified as F. bocagei and F. mechowii are heterospecific and the lineages were also required to be monophyletic with posterior probability (PP) ≥ 0.50. For better understanding of differentiation between the putative species, we calculated average pairwise Kimura two-parameter (K2P) distances (Kimura, 1980) separating them.
The short sequences of museum specimens, not included in the CYTB tree, were classified into the CYTB lineages using an evolutionary placement algorithm (EPA; Berger et al., 2011) implemented in RaxML v8.2.10 (Stamatakis, 2014). In short, the algorithm attempts to place query sequences one by one on the pre-specified phylogenetic tree and outputs a table containing the potential placements (i.e., tree branches) with their likelihood weights. We set EPA to place every short CYTB sequence separately on the MCC tree from MrBayes analysis and for each of them we calculated relative probabilities of belonging to different lineages. The probabilities are sums of likelihood weights of placements on branches belonging to the lineages. R functions used for post-processing of EPA outputs are available at https://github.com/onmikula/epatools.
The inference of the nuclear phylogenetic tree was also accomplished in MrBayes using concatenated sequences of all five loci. Each locus was set as a separate partition with its own nucleotide substitution model selected by ModelFinder as in the CYTB analysis. The models selected were HKY + F for DHCR and SMO, HKY + F + G4 for FGB, GTR + F for RAG1, and GTR + F + G4 for TRPV (Hasegawa et al., 1985; Tavaré, 1986; Yang, 1994). The nuclear sequences were analysed as unphased with heterozygous states treated as ambiguous. MrBayes was run four times independently for 5 × 106 generations with sampling every 5000th generation. The convergence of MCMC was checked and the posterior samples were summarized as in the CYTB analysis.
Karyotype of F. darlingi from Nsanje
The karyotype of F. darlingi from the Nsanje population was established from a blood sample taken from one male kept at the University of Duisburg-Essen (F2 offspring of wild-caught mole-rats). Lymphocytes in whole blood cultures were stimulated to enter the cell cycle using phytohemagglutinin L (PAN Biotech, 3 μg/mL) for 2 days at 37 °C. Mitotic cells were arrested using colcemid (Ciba, 80 ng/mL). Preparation and Giemsa staining followed standard protocols. Chromosomes in C-metaphases were examined. Altogether, 12 mitotic cells were analysed.
Reproduction, postnatal development, and sexual dimorphism of F. darlingi from Nsanje
Families of F. darlingi from the Nsanje population were monitored in the vivaria of the University of South Bohemia in České Budějovice and the University of Duisburg-Essen. The mole-rats were kept in glass terraria of varying sizes with horticultural peat (České Budějovice) or saw dust (Essen) as substrate. Ceramic flowerpots were offered as nest chambers, and filter paper as nesting material. Room temperature was 25 ± 1 °C, relative air humidity 40–60%, and light regime 12D/12L. The animals were fed with potatoes, sweet potatoes, carrots, apples, and commercial rodent pellets thrice a week.
Data on reproduction and postnatal development were collected for individuals from 14 families. We recorded litter size and inter-birth intervals as a proxy for the length of gestation (c.f. Burda, 1989, 1990). For some pups, we specifically monitored their development after birth, including body mass (g, measured every 3 days), the presence of fur and externally visible incisors, and the opening of eyes, as well as specific behaviours (eating solids, leaving the nest for the first time, starting to spar with other family members).
We calculated linear mixed models with the body mass of pups (g) as the dependent variable, litter size as a fixed factor, and litter number as a random factor with the aid of the package lme4 (Bates et al., 2022) in Rstudio (Rstudio Team, 2020). Gaussian distribution of the dependent variable and the model residuals were both tested with a Shapiro–Wilk test for normality (p > 0.05).
Body weight W at time t and growth parameters fitted to a Gompertz growth curve were estimated in a total of 73 individuals (32 males, 41 females) for which approximately adult body mass was reached (i.e., at least 90 g and/or not gaining weight over a period of 4 weeks) using the following formula:
with A being the asymptotic body mass (g), K the growth constant (days−1), and I the inflection point. The maximum growth rate (g × days−1) was calculated as K × A × e−1 (Begall, 1997). The mean age of individuals for which growth parameters have been calculated was 8.1 ± 4.5 years (range: 1–16.4 years). Growth parameters including maximum growth rate were analysed with generalized linear regression models with family as a random factor and sex and breeding status as explanatory variables.
We visualized growth trajectories of males and females of both status groups (breeders and non-breeders) over the course of 10 years (520 weeks). Weekly mean values were taken into account for a group according to sex and breeding status, if body weights for at least three individuals of the respective status group had been collected.
Results
Wild-caught F. darlingi from Nsanje
Wild-caught mole-rats were of dark grey colour and displayed relatively large white head spots of different sizes and shapes (see Fig. 2). Males were larger than females (Table 1) and one of the females was pregnant with three foetuses. Other information on morphology and reproductive condition of free-living individuals can be found in Supplementary Information 1.
Selected Fukomys mole-rats from Malawi and adjacent regions. Shown are representatives of F. whytei from southern Tanzania (top left), nominate F. darlingi from Zimbabwe (top right), and F. darlingi from Nsanje in southern Malawi (below left: in the field on the day of capture; below right: captive family at the University of South Bohemia in České Budějovice). Photos by Tim Jackson and R. Šumbera
Karyotype of F. darlingi from Nsanje
The diploid chromosome number of F. darlingi from Nsanje was 2n = 54 with 23 metacentric chromosomes (including the single X chromosome in males) and 31 acro/telocentric chromosomes (including the single Y chromosome in males). The fundamental number was 78 with the autosomal fundamental number (aFN) being 76 (Fig. 3).
Phylogenetic position of F. darlingi from Nsanje and phylogeny of the genus Fukomys
The CYTB tree (Fig. 4) contained 15 lineages delimited by the branch-cutting method, which represent already recognized species of Fukomys (F. foxi, F. zechi, F. mechowii, F. bocagei, F. livingstoni, F. hanangensis, F. whytei, F. darlingi, F. damarensis, and F. micklemi), but also new putative species (“Chimbolo” from central Zambia, “Chinyingi” and “Watopa” from western Zambia, “Ndawambe” from the border of Zambia and Malawi, and “Viphya” from northern Malawi). The topology of the MCC tree showed the pair of haplotypes from Nsanje belong to F. darlingi lineage with PP = 1.00. Mole-rats from Nsanje together with mole-rats from Gorongosa in Mozambique (PP = 1.00) formed an internal lineage within F. darlingi. This is in accordance with their geographical distribution, as Gorongosa is the nearest locality to Nsanje. Average K2P distances between F. darlingi and other CYTB lineages ranged from 0.0688 (F. damarensis) to 0.1742 (F. foxi). The average K2P distances observed within the F. darlingi lineage were lower: 0.0299 in F. darlingi as a whole, 0.0029 within the Nsanje population, and 0.0285 between the Nsanje population and the remaining individuals assigned to F. darlingi. Such internal variation is comparable to other lineages with larger geographical distributions, e.g., F. micklemi (0.0179) or F. mechowii (0.0374). For the complete matrices of pairwise distances between CYTB lineages and between individuals of F. darlingi lineage, see Supplementary Information 3. All short CYTB sequences of museum samples were placed with very high probability (> 0.99) into F. darlingi. This data, together with other museum records of mole-rats from the area, indicate that this species is mainly distributed across the Mashonaland plateau in Zimbabwe and the lowland regions of Mozambique between the Zambezi and Save rivers (see Fig. 1).
Mitochondrial phylogeny of Fukomys mole-rats. MCC tree estimated from the CYTB dataset with coloured lineages delimited by the branch-cutting method. The colours of the CYTB lineages match those shown in the range map and the nuclear tree (Figs. 1 and 5). The tip labels contain specimen IDs and those belonging to F. darlingi also locality of origin
The nuclear phylogenetic tree only partially matched its CYTB counterpart (Fig. 5). Most of the CYTB lineages are found also in the nuclear tree, but some of them were intermixed, namely those identified with species F. damarensis and F. micklemi. The monophyly of major CYTB clades, including damarensis-micklemi-“Chimbolo”, was largely supported by nuclear data, but their relationships sometimes differed between the trees. For instance, the darlingi lineage has different closest relatives in both trees: the clade damarensis-micklemi- “Chimbolo”- “Chinyingi”- “Watopa” in CYTB tree, but the clade whytei-hanagensis-“Viphya”- “Ndawambe” in the nuclear phylogeny. Nevertheless, the mole-rats from Nsanje are again recovered as a part of the F. darlingi with a high support (PP = 1.00). Although they occupy the periphery of the species’ range, they belong to a lineage deeply nested within F. darlingi (PP = 0.98).
Nuclear phylogeny of Fukomys mole-rats. MCC tree estimated from nuclear datasets of five genes. Tip labels are colour-matched with those shown in the range map and the mitochondrial tree (Figs. 1 and 4). Each tip name contains the sample ID, the name of the respective CYTB lineage, and in the case of samples from F. darlingi clade also the name of the collection locality
Reproduction, postnatal development, and sexual dimorphism of F. darlingi from Nsanje
Fukomys darlingi from Nsanje bred throughout the year in both labs. Based on observations from regularly breeding families, the mean inter-birth interval was 111 ± 4.7 days (n = 80 pregnancies of 19 females) and mean litter size 2.3 ± 1.1 (n = 138 litters) (Table 1). Nevertheless, average litter size was larger (3.0 ± 1.0, n = 17) in the first years after the establishment of the species in České Budějovice (Jerkovičová 2010).
On the day of birth, the pups were of pink colour and, if at all, only sparsely haired with a clear whitish spot on the head, which allowed for individual identification. The eyes were closed while the protruding extrabuccal incisors were already apparent. The mean body mass of neonates was 9.8 ± 1.7 (n = 108 pups from 50 litters) (Table 1). Body mass of pups was not influenced by litter size (t = − 1.003; d.f. = 1; P = 0.318; n = 108 pups, 50 litters).
Pups left the nest at an age of about 2 weeks and also started to consume solid food at that time. Eyes opened at an age of 7 weeks and at the same age pups started sparring with other juveniles. Their postnatal development was slow; see Fig. 6 for the growth trajectories of 32 males (13 breeders, 19 non-breeders) and 40 females (13 breeders, 27 non-breeders) over the course of 520 weeks. Sex was found to have a significant effect on asymptotic body mass A, inflection point I, and maximum growth rate, but not on the growth constant K (Fig. 7, Table 2). Breeding status was shown to have an influence on the asymptotic body mass, but not on any other parameter. The combined effect of sex and breeding status exerted statistically significant effects on all parameters (K, I, and maximum growth rate), but not on the asymptotic body mass. The mean growth during the first 80 days of life was on average 0.37 ± 0.08 g/day with significant differences between the four status groups (one-way ANOVA, F3,64 = 5.47; p = 0.002). A Tukey HSD test revealed a significant difference between male breeders (0.43 ± 0.06 g/day) and female non-breeders (0.33 ± 0.07 g/day; padj < 0.0015); differences between the other multiple comparisons were not significant. Adult (≥ 18 months) body mass was determined to be 114 ± 15.4 g (n = 51) in captive females and 153.6 ± 22.6 (n = 45) in captive males and hence somewhat higher than in wild Nsanje mole-rats (Table 1).
Growth in captive Fukomys darlingi from the Nsanje population. Mean body mass (g) calculated weekly for individuals belonging to one of four groups based on sex and breeding status (non-breeding female NB, n = 27; breeding female B, n = 13; non-breeding male NB, n = 19; breeding male B, n = 13) over a period of 10 years. Gompertz growth parameters have been estimated for each individual separately, and thereafter, mean values have been calculated for each group. These parameters have been used for modelling the solid lines
Breeding females reproduced up to an age of at least 14 years, but the respective animals were still breeding at the time this manuscript was written. Nsanje mole-rats can still be successfully mated for the first time at an age of 8–10 years and are generally long-lived, as is typical for the genus Fukomys. The maximum life span is at least 19 years, because a female trapped in the field in 2005, at an estimated age of 1 year, died in January 2023 in the vivarium at the University of South Bohemia.
Discussion
Phylogeny, diversity, and biogeography of F. darlingi
We demonstrate that mole-rats from Nsanje form a nested lineage within F. darlingi and that a species or subspecific status for these animals is unwarranted considering the genetic and karyological data presented. The Nsanje population was not even found to be monophyletic in the concatenated nuclear tree and based on CYTB data its average distance from the rest of F. darlingi is comparable to the overall variability in the species. The remarkable morphological and reproductive differences between F. darlingi populations thus do not align with phylogenetic distance, and instead correspond to intraspecific variation. We thus recommend retaining F. darlingi as a monotypic species, as is the current consensus (Honeycutt, 2016; Monadjem et al., 2015).
In the nuclear phylogeny, F. darlingi forms the sister lineage to the whytei-hanangensis-“Viphya”-“Ndawambe” clade (PP = 0.99) (Fig. 4). This relationship would make the species a part of an eastern-African radiation of Fukomys, which is nested in the major south-eastern African clade and whose species occupy savannah environments from Tanzania to north-eastern Zambia, Malawi, and Zimbabwe. At the same time, this inferred sister group relationship makes F. darlingi a clear example of mitonuclear discordance, because in the CYTB tree, the lineage is supported unambiguously (PP = 1.00) as a sister of the clade formed by five lineages corresponding to F. damarensis and F. micklemi s.l. (Fig. 4). The discordance could be caused by incomplete lineage sorting, which is especially common in radiations that experienced a rapid succession of ancestral speciation events. It could also be caused by introgression from the ancestor of the micklemi-damarensis clade into F. darlingi (or vice versa). Both, F. darlingi and F. damarensis are distributed mainly south of the Zambezi River. If assuming the same for their common ancestor and in case their split post-dated the branching of F. darlingi from the whytei-hanangensis-“Viphya”-“Ndawambe” clade, such an introgression scenario appears feasible.
Given their nested position among F. darlingi lineages and because all other records of F. darlingi are from south of the Zambezi River in eastern Zimbabwe and adjacent parts of Mozambique, it appears likely that the ancestors of the Nsanje form of F. darlingi crossed the Zambezi River to colonize southern Malawi. Although mole-rats generally can swim well due to their cylindrical body and broad hands and feet (Hickman, 1978), we do not expect that they were able to actively cross such a large river, especially at its lower reaches. Alternatively, changes of the river course, which might have repeatedly isolated and released parts of mole-rat populations, could have enabled the ancestors of the Nsanje mole-rats to successively overcome this barrier. Indeed, the Zambezi and its tributaries are known for the occurrence of marked ox bows in the river course. A similar type of dispersal has been suggested to explain how annual killifish of the genus Nothobranchius, i.e., fishes which do not inhabit streams and are very poor swimmers, can cross the lower reaches of large rivers in the lowland flood plains of Mozambique (Bartáková et al., 2015).
We would like to briefly touch on the cases of two purported species of social mole-rats that are no longer recognized by taxonomists. Both were described from the vicinity of Beira in the lowland areas of Mozambique, namely Georychus beirae Thomas & Wroughton, 1907 (later Cryptomys beirae – see Roberts, 1951) and Cryptomys zimbitiensis (Roberts, 1946). Animals originally assigned to these species are virtually indistinguishable from one another (De Graaff, 1964) and both are currently synonymized with F. darlingi (Happold, 2013; Mammal Diversity Database, 2022). These mole-rats have been described as being slightly larger than nominate F. darlingi with a somewhat lighter, more yellowish fur colouration (De Graaff, 1964). Apart from their occurrence around Beira, they were also reported from Gorongosa (Roberts, 1951; see De Graaff, 1964). Our results clearly demonstrate that mole-rats from Gorongosa are closely related to those from Nsanje. Concordantly, the description of beirae by Roberts (1951) matches the body measurements (head and body length, hind foot) of mole-rats from Nsanje including the presence of a white patch on the head. Both populations are larger than nominate F. darlingi from the Zimbabwean highlands (Roberts, 1951; Thomas, 1895). Inspection of the holotypes of F. darlingi (NHMUK 1895.7.16.4) and the beirae form (NHMUK 1907.6.2.98) did not reveal any notable differences in their coat colouration (see Supplementary Information 4). Considering these data, it seems appropriate to retain all specimens denoted as beirae and zimbitiensis within F. darlingi.
How many Fukomys species inhabit Malawi?
Although it was originally suggested that common mole-rats in Malawi belong to a single species, which was referred to as Cryptomys hottentotus (Ansell & Dowsett, 1988), it becomes clear that the country is home to at least four well-defined lineages of Fukomys, most likely distinct species (Figs. 1, 4, and 5). The northern parts of Malawi are occupied by F. whytei, whereas in the southernmost regions of Malawi, F. darlingi is present. Two other lineages provisionally named “Ndawambe” and “Viphya” occur in the central part of the country. Whereas mole-rats from Viphya seem to be related to F. whytei in both the CYTB and nuclear dataset, the position of those from Ndawambe is not clear (Figs. 4 and 5). Mole-rats from Mzuzu in northern Malawi (see Faulkes et al., 2010) seem to be almost identical to the animals from Viphya according to our unpublished CYTB data. For sure, these relatively isolated lineages deserve further study. Ansell and Dowsett (1988) proposed that F. mechowii (Peters, 1881), the giant mole-rat, which is abundant in northern Zambia, also occurs in northern Malawi. However, the single specimen that this assumption was based on exhibited a head spot, which is typically absent in this species (Caspar et al., 2021b). No further records of F. mechowii from Malawi have been noted since then, and it appears that the species’ eastern range is limited by the Zambian Muchinga Escarpment and the Luangwa River system (see Caspar et al., 2021b). Thus, its range should not extend into Malawi. In case F. mechowii is indeed absent from Malawi and the mole-rat populations from Ndawambe and Viphya are recognised as species in future analyses, the bathyergid fauna of the country will consist of five species: four social Fukomys and the solitary silvery mole-rat Heliophobius argenteocinereus.
Karyotypic differentiation within F. darlingi
The karyotype of F. darlingi from Nsanje greatly resembles that of the nominate form sampled at Goromonzi (Zimbabwe). Both possess a diploid number of 2n = 54 (Aguilar, 1993, this study), but there are deviations in the autosomal fundamental number (aFN = 76 in this study, aFN = 80 in Aguilar’s study). Aguilar (1993) reports three pairs of submetacentric chromosomes that could not be identified in our sample. Because it is difficult to differentiate the submetacentric from the acrocentric chromosomes in the karyogram presented by Aguilar (1993), it might well be that there is no difference at all in the karyotypes of Nsanje and nominate F. darlingi.
Variation in body size in F. darlingi
One of the most remarkable differences between populations of F. darlingi from Nsanje and the nominate form studied at Goromonzi lies in their body mass. Wild-caught mole-rats from Nsanje are almost twice as large as individuals from Goromonzi used to establish a captive breeding group at the University of Pretoria (Bennett et al., 1994; this study, Table 1, Fig. 8). Adults of the nominate form from Goromonzi exhibit a mean body mass of 70.6 g (± 7.5 g; n = 8) in females and 92.6 g (± 12.4 g; n = 10) in males (Bennett et al., 1994; Gabathuler et al., 1996; Herbst & Bennett, 2001), while it was found to be 114 g (± 15.4 g; n = 51) in Nsanje females and 153.6 g (± 22.6, n = 45) in Nsanje males older than 18 months (Table 1). This is also the case for Zimbabwean F. darlingi (Bennett et al., 1997). Interestingly, Genelly (1965) reported body mass of males 110 g (80–156, n = 16) and females 87 g (50–114, n = 13) from a mole-rat population in Harare, the type locality of F. darlingi (and not far from Goromonzi). It seems that mole-rats from Goromonzi are the smallest representatives of F. darlingi, and in fact among the smallest of all Fukomys currently known.
Adult body mass distribution of Fukomys darlingi from Zimbabwe (nominate form, n = 18) and southern Malawi (Nsanje form, n = 96). Note the larger body mass and greater sexual dimorphism in animals from Nsanje. Data on F. darlingi individuals from Goromonzi were compiled from Bennett et al. (1994), Gabathuler et al. (1996), and Herbst and Bennett (2001); animals were deemed adult when identified as breeders or when being heavier as the same-sex breeder in the respective family. Nsanje mole-rats were classified as adults at an age of 18 months
Pelage colouration
Happold (2013) described the nominate form of F. darlingi as an animal with a longitudinal white stripe on the ventrum which may extend along the full medial axis of the body. Other authors have not mentioned such stripes, nor did we find them in museum specimens of the species, including the holotype. It is also absent in the Nsanje form, but some individuals had white markings stretching from the chin down to the throat. In other species of Fukomys, such as F. damarensis, F. anselli, and F. micklemi, the presence of a medial abdominal stripe is also a polymorphic trait (pers. obs.). Size and shape of the head spots in the Nsanje mole-rats correspond well to detailed descriptions of this characteristic available for F. darlingi from Harare (Genelly, 1965). Animals from Goromonzi also exhibit a large head spot (see Fig. 2). Compared to the slate grey animals from Nsanje, F. darlingi from Goromonzi typically have a more brownish colour (Supplementary Information 4). Nevertheless, we may assume that fur colouration bears no taxonomic value because large variation in pelage colour ranging from light brown to dark grey was found among different families caught at the same locality and even within families of F. darlingi from Harare (Genelly, 1965). In addition, brownish colored juveniles born to grey-furred parents were observed in two breeding groups at the lab at the University Duisburg-Essen (pers. obs.). The brown colouration of these juveniles turns to slate grey at an age of about 18 months. In other captive families of Nsanje mole-rats, such a pattern is not apparent. While some species of Fukomys, such as F. anselli and F. mechowii, exhibit a well-documented ontogenetic colour change (Burda, 1989; Scharff et al. 1999), this trait has so far not been described in F. darlingi. In old animals (> 12 years), we noticed that the fur, particularly on the head, progressively turns pale.
Ontogeny and reproduction
In several reproductive parameters, mole-rats from Nsanje resemble similarly sized Fukomys species, especially F. damarensis, more than nominate conspecifics. This is the case, for instance, for neonate body mass which is 8–9 g in F. damarensis (Bennett & Jarvis, 2004; Bennett et al., 1991), but 6.9–8.2 g in F. darlingi from Goromonzi. The difference is also remarkable in mean litter size, which is 3.0 (1–6) in F. damarensis (Bennett & Jarvis, 2004) and 2.3 pups in the Nsanje F. darlingi studied here, but only 1.7 in F. darlingi from Goromonzi (Bennett et al., 1994). Whereas smaller litter size in F. darlingi from Goromonzi could be attributed to their smaller body size it may also be an artefact created by the low sample size of just n = 2 (Table 1) for this population. Although litter size in Nsanje mole-rats based on our long-term records is relatively small (2.3), it was larger (3.0) in the beginning of the history of our breeding in České Budějovice when a detailed study on reproduction was carried out (Jerkovičová, 2010). We assume that the smaller average litter sizes recovered in this study are caused by including a large number of usually smaller litters of young breeding females from recently established families in Essen. It can be hypothesized that average litter size may increase with parity in regularly breeding females similarly to, for instance, F. mechowii (Scharff et al., 1999). Behavioural parameters such as leaving the nest, starting to consume solid food, and the emergence of agonistic interactions between pups are comparable to nominate F. darlingi (see Table 1).
The most pronounced inter-population difference within F. darlingi seems to be the length of pregnancy (i.e., inter-birth interval). Assuming that the minimum inter-birth interval corresponds to the maximum length of pregnancy (as in F. anselli, cf. Burda, 1989, 1990), we estimate the length of gestation in F. darlingi from Nsanje to be about 111 days. This fits the typical Fukomys pattern: 98 days in F. anselli (Begall & Burda, 1998; Burda, 1989); 112 days in F. mechowii (Scharff et al., 1999); 78–92 days in F. damarensis (Bennett & Jarvis, 1988, 2004). We found much longer pregnancies in the Nsanje form than were reported for F. darlingi from Zimbabwe. For these animals, Bennett et al. (1994) and Herbst and Bennett (2001) reported gestation lengths of just about 60 days (Table 1). Due to very low sample sizes reported, it is hard to evaluate if this is a real inter-population difference or, alternatively, an observational artefact. Observed mating events do not necessarily lead to successful fertilisation and in addition, it is known that females of Fukomys engage in copulations even when already pregnant (Burda, 1989), making estimates based on this behavioural measure dubious. It should also be mentioned that such great intraspecific differences in the length of pregnancy would be very unusual for small mammals (Kiltie, 1982). Interestingly, the reported lowest values for intervals between litters or timespans from pairing to parturition in F. darlingi from Goromonzi correspond well to mean inter-birth interval in our study (Bennett et al., 1997; Greeff & Bennett, 2000). This may indicate that the length of pregnancy has been underestimated for the Zimbabwean population of the species. Nevertheless, this topic surely deserves further study.
A comparison of Gompertz growth parameters (Table 3) reveals that F. darlingi originating from Nsanje exhibit the lowest growth constants and the lowest maximum growth rates (0.19 g/day) of all bathyergids. This is consistent with our observations from the laboratory, indicating that these animals may still grow at an age of 2 years, when congeneric species are considered to be fully adult. Since many of the reported species growth parameters have asymptotic values much lower than the actual body masses, it is obvious that the calculations were based on incomplete records (i.e., animals not fully grown) which leads to an overestimation of the growth constants (see Begall, 1997, for details). The growth constants obtained for F. darlingi from Nsanje are slightly lower than those of other Fukomys species for which reliable data are available (e.g. F. anselli – Begall & Burda, 1998, F. mechowii – Scharff et al., 1999), but still in the same order of magnitude (Table 3).
Differences in growth parameters between the sexes (as well as between breeders and non-breeders) are evident also in animals older than 80 days reflecting sexual size dimorphism typical for Fukomys mole-rats (Caspar et al., 2021c). The difference between the mean growth rates during the first 80 postnatal days of (future) male breeders compared to female non-breeders might be an artefact of the selection regime for breeders. As the initial male population was very small (n = 4), rather large fast-growing males of the first generation of offspring might have been preferentially picked as founders of new families.
The only previously available estimate for the life span of F. darlingi was 7 years (Greeff & Bennett, 2000). Our data suggest that the maximum life span of the species is much longer, i.e., at least 19 years. This corresponds better to data recorded of other Fukomys species, which may live to an age beyond 20 years (Begall et al., 2021; Dammann pers. com.; Fang et al., 2014).
What causes inter-population differences in F. darlingi?
Both inter- and intraspecific differences in various morphological parameters can frequently be related to the ecological conditions the respective populations face. One of the best-known ecological rules explaining differences in body mass is Bergmann’s rule, proclaiming that within clades of endothermic vertebrates, those living in colder environments are larger than those deriving from warmer habitats (Bergmann, 1847). The range of F. darlingi indeed covers areas differing markedly in ambient temperatures, which are chiefly coupled to altitude. For instance, Nsanje is situated close to sea level, while Goromonzi is located over 1000 m a.s.l. Thus, the intraspecific body mass pattern fits the inverse to Bergmann’s rule, with smaller mole-rats occupying higher altitudes (Table 1). Among subterranean mammals, the inverse to the Bergman rule has been described already in the South American tuco-tucos of the genus Ctenomys (Medina et al., 2007). Unfortunately, due to lack of data, it is hard to estimate which other factor such as different soil or food characteristics may play a role in shaping such dramatic differences in body mass.
In F. darlingi, it appears that the variability in body mass is indeed responsible for many inter-population differences that have been characterized in various studies. Apart from parameters related to reproduction, another example is the ability to keep a stable core body temperature. Whereas smaller F. darlingi from the nominate population lack this capacity at low ambient temperatures and show signs of heterothermy (Bennett et al., 1993), their conspecifics from Nsanje are truly homeothermic keeping their body temperature stable across a substantial range of experimental ambient temperatures (Zemanová et al., 2012; but see another study on F. darlingi from Goromonzi in which mole-rats do not show such remarkable decrease of Tb in lowest measured Ta = 18 °C, Boyles et al., 2012). Although Zemanová et al. (2012) speculated that the animals tested by Bennett et al. (1993) were not fully grown, the fact that some of them were already able to reproduce demonstrates that deviations in body mass cannot be attributed to ontogeny. This remarkable intraspecific difference in thermal biology has been explained by the overall lowered ability to defend stable Tb in smaller animals with worse surface body mass ratio (Zemanová et al., 2012).
Conclusion
In our study, we took an integrative approach to analyse the phylogeny and biology of an isolated lowland population of social African mole-rats from southern Malawi. Our multi-locus phylogenetic analysis based on samples of both fresh and museum-conserved tissues convincingly demonstrated that the mole-rats from Nsanje are Mashona mole-rats, Fukomys darlingi, a species otherwise distributed across the highlands of Zimbabwe and lowland regions of Mozambique. Karyological data align with this diagnosis. Overall, the bathyergid mole-rat fauna of Malawi was shown to likely encompass at least four species of Fukomys besides one of the genus Heliophobius. We also document an instance of mitonuclear discordance in major lineages of Fukomys.
We further present information on various life history parameters of the Nsanje population of F. darlingi. Although there are some remarkable inter-population differences between the Nsanje and the nominate form of these mole-rats in reproductive and physiological characteristics, these deviations can largely be attributed to dramatic differences in body mass between animals from different localities. Future studies should address why lowland populations of F. darlingi from warm humid areas are notably larger than those from cooler highland environments.
Availability of data and materials
GPS of localities, museum voucher numbers, GenBank Accession numbers, and other details about specimens included in analyses are provided in Supplementary Information 2. New DNA sequences were uploaded to GenBank and their accession numbers (OQ559424-OQ559475, OQ442881–OQ442896, OQ442843–OQ442861, OQ559483–OQ559501, OQ559505–OQ559523, OQ442862–OQ442880) are in Supplementary Information 2.
Code availability
Not applicable.
References
Aguilar, G. H. (1993). The karyotype and taxonomic status of Cryptomys hottentotus darlingi (Rodentia: Bathyergidae). African Zoology, 28(4), 201–204.
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723. https://doi.org/10.1109/TAC.1974.1100705
Ansell, W. F. H., & Dowsett, R. (1988). Mammals of Malawi: An annotated check list and atlas. Trendrine Press.
Bartáková, V., Reichard, M., Blažek, R., Polačik, M., & Bryja, J. (2015). Terrestrial fishes: Rivers are barriers to gene flow in annual fishes from the African savanna. Journal of Biogeography, 42(10), 1832–1844. https://doi.org/10.1111/jbi.12567
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2022). Lme4: Linear mixed-effects models using Eigen and S4. R Package Version, 1, 1–30.
Begall, S. (1997). The application of the Gompertz model to describe body growth. Growth, Development & Aging, 61, 61–67. https://doi.org/10.1371/journal.pone.0178691
Begall, S., & Burda, H. (1998). Reproductive characteristics and growth rate in the eusocial Zambian common mole-rat (Cryptomys sp., Bathyergidae). Zeitschrift für Säugetierkunde, 63(5), 297–306. https://doi.org/10.1515/mamm.1999.63.2.217
Begall, S., Burda, H., & Šumbera, R. (2018). Graumulle – Cryptomys und Fukomys. VerlagsKG Wolf.
Begall, S., Nappe, R., Hohrenk, L., Schmidt, T. C., Burda, H., Sahm, A., Szafranski, K., Dammann, P., & Henning, Y. (2021). Life expectancy, family constellation and stress in giant mole-rats (Fukomys mechowii). Philosophical Transactions of the Royal Society B, 376(1823), 20200207. https://doi.org/10.1098/rstb.2020.0207
Bennett, N. C., & Faulkes, C. G. (2000). African mole-rats: Ecology and eusociality. Cambridge University Press.
Bennett, N. C., & Jarvis, J. U. M. (2004). Cryptomys damarensis. Mammalian Species, 756, 1–5. https://doi.org/10.1644/756
Bennett, N. C., Jarvis, J. U. M., & Cotterill, F. P. D. (1993). Poikilothermic traits and thermoregulation in the Afrotropical social subterranean Mashona mole-rat (Cryptomys hottentotus darlingi) (Rodentia: Bathyergidae). Journal of Zoology, 231(2), 179–186. https://doi.org/10.1111/j.1469-7998.1993.tb01910.x
Bennett, N. C., Jarvis, J. U. M., & Cotterill, F. P. D. (1994). The colony structure and reproductive biology of the afrotropical Mashona mole-rat, Cryptomys darlingi. Journal of Zoology, 234(3), 477–487. https://doi.org/10.1111/j.1469-7998.1994.tb04861.x
Bennett, N. C., & Jarvis, J. U. M. (1988). The social structure and reproductive biology of colonies of the mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae). Journal of Mammalogy, 69(2), 293–302. https://doi.org/10.2307/1381379
Bennett, N. C., Faulkes, C. G., & Spinks, A. C. (1997). LH responses to single doses of exogenous GnRH by social Mashona mole-rats: A continuum of socially induced infertility in the family Bathyergidae. Proceedings of the Royal Society of London, Series B: Biological Sciences, 264(1384), 1001–1006. https://doi.org/10.1098/rspb.1997.0138
Bennett, N. C., Jarvis, J. U. M., Aguilar, G. H., & McDaid, E. J. (1991). Growth and development in six species of African mole-rats (Rodentia: Bathyergidae). Journal of Zoology, 225(1), 13–26. https://doi.org/10.1111/j.1469-7998.1991.tb03798.x
Berger, S. A., Krompass, D., & Stamatakis, A. (2011). Performance, accuracy, and web server for evolutionary placement of short sequence reads under maximum likelihood. Systematic Biology, 60(3), 291–302. https://doi.org/10.1093/sysbio/syr010
Bergmann, C. (1847). Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien.
Bishop, J. M., Jarvis, J. U. M., Spinks, A. C., Bennett, N. C., & O’Rian, C. (2004). Molecular insight into patterns of colony composition and paternity in the common mole-rat Cryptomys hottentotus hottentotus. Molecular Ecology, 13(5), 1217–1229. https://doi.org/10.1111/j.1365-294X.2004.02131.x
Boyles, J. G., Verburgt, L., McKechnie, A. E., & Bennett, N. C. (2012). Heterothermy in two mole-rat species subjected to interacting thermoregulatory challenges. Journal of Experimental Zoology Part a: Ecological Genetics and Physiology, 317, 73–82. https://doi.org/10.1002/jez.723
Burda, H. (1989). Reproductive biology (behaviour, breeding, and postnatal development) in subterranean mole-rats, Cryptomys hottentotus (Bathyergidae). Zeitschrift für Säugetierkunde, 54(6), 360–376.
Burda, H. (1990). Constraints of pregnancy and evolution of sociality in mole-rats with special reference to reproductive and social patterns in Cryptomys hottentotus (Bathyergidae, Rodentia). Journal of Zoological Systematics and Evolutionary Research, 28(1), 26–39. https://doi.org/10.1111/j.1439-0469.1990.tb00362.x
Burda, H., Zima, J., Scharff, A., Macholan, M., & Kawalika, M. (1999). The karyotypes of Cryptomys anselli sp. nova and Cryptomys kafuensis sp. nova: New species of the common mole-rat from Zambia (Rodentia, Bathyergidae). Zeitschrift für Säugetierkunde, 64, 36–50.
Burland, T. M., Bennett, N. C., Jarvis, J. U. M., & Faulkes, C. G. (2002). Eusociality in African mole-rats: New insights from patterns of genetic relatedness in the Damaraland mole-rat (Cryptomys damarensis). Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1495), 1025–1030. https://doi.org/10.1098/rspb.2002.1978
Caspar, K. R., Burda, H., & Begall, S. (2021b). Fukomys mechowii (Rodentia: Bathyergidae). Mammalian Species, 53(1011), 145–159.
Caspar, K. R., Heinrich, A., Mellinghaus, L., Gerhardt, P., & Begall, S. (2021a). Evoked auditory potentials from African mole-rats and coruros reveal disparity in subterranean rodent hearing. Journal of Experimental Biology, 224(22), jeb243371. https://doi.org/10.1242/jeb.243371
Caspar, K. R., Müller, J., & Begall, S. (2021c). Effects of sex and breeding status on skull morphology in cooperatively breeding Ansell’s mole-rats and an appraisal of sexual dimorphism in the Bathyergidae. Frontiers in Ecology and Evolution, 9, 638754. https://doi.org/10.3389/fevo.2021c.638754
Contreras, L. C., & McNab, B. K. (1990). Thermoregulation and energetics in subterranean mammals. Evolution of subterranean mammals at the organismal and molecular levels. Progress in Clinical and Biological Research, Alan R. Liss Inc 231-250
De Graaff, O. (1964). A systematic revision of the Bathyergidae (Rodentia) of Southern Africa. Ph.D. thesis, Pretoria: University of Pretoria.
Drummond, A. J., & Bouckaert, R. R. (2015). Bayesian evolutionary analysis with BEAST. Cambridge University Press.
Dvořáková, V., Hrouzková, E., & Šumbera, R. (2016). Vocal repertoire of the social Mashona mole-rat (Fukomys darlingi) and how it compares with other mole-rats. Bioacoustics, 25(3), 253–266. https://doi.org/10.1080/09524622.2016.1141117
Faulkes, C., Mgode, G. F., Le Comber, S. C., & Bennett, N. C. (2010). Cladogenesis and endemism in Tanzanian mole-rats, genus Fukomys: (Rodentia Bathyergidae): A role for tectonics? Biological Journal of the Linnean Society, 100(2), 337–352. https://doi.org/10.1111/j.1095-8312.2010.01418.x
Fang, X., Seim, I., Huang, Z., Gerashchenko, M. V., Xiong, Z., Turanov, A. A., Zhu, Y., Lobanov, A. V., Fan, D., Yim, S. H., Yao, X., Ma, S., Yang, L., Lee, S. G., Kim, E. B., Bronson, R. T., Šumbera, R., Buffenstein, R., Zhou, X., … Gladyshev, V. N. (2014). Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Reports, 8(5), 1354–1364. https://doi.org/10.1016/j.celrep.2014.07.030
Faulkes, C. G., Bennett, N. C., Bruford M. W., O’Brien, H. P., Aguilar, G. H., & Jarvis, J. U. M. (1997). Ecological constraints drive social evolution in the African mole–rats, Proceedings of the Royal Society of London. Series B: Biological Sciences, 264(1388), 1619–1627. https://doi.org/10.1098/rspb.1997.0226
Faulkes, C. G., Mgode, G. F., Archer, E. K., & Bennett, N. C. (2017). Relic populations of Fukomys mole-rats in Tanzania: Description of two new species F. livingstoni sp. nov. and F. hanangensis sp. nov. PeerJ, 5, e3214. https://doi.org/10.7717/peerj.3214
Faulkes, C. G., & Bennett, N. C. (2013). Plasticity and constraints on social evolution in African mole-rats: Ultimate and proximate factors. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 368(1618), 20120347. https://doi.org/10.1098/rstb.2012.0347
Faulkes, C. G., Bennett, N. C., Cotterill, F. P. D., Stanley, W., Mgode, G. F., & Verheyen, E. (2011). Phylogeography and cryptic diversity of the solitary-dwelling silvery mole-rat, genus Heliophobius (family: Bathyergidae). Journal of Zoology, 285(4), 324–338. https://doi.org/10.1111/j.1469-7998.2011.00863.x
Faulkes, C. G., Verheyen, E., Verheyen, W., Jarvis, J. U. M., & Bennett, N. C. (2004). Phylogeographical patterns of genetic divergence and speciation in African mole-rats (Family: Bathyergidae). Molecular Ecology, 13(3), 613–629. https://doi.org/10.1046/j.1365-294X.2004.02099.x
Gabathuler, U., Bennett, N. C., & Jarvis, J. U. M. (1996). The social structure and dominance hierarchy of the Mashona mole-rat, Cryptomys darlingi (Rodentia: Bathyergidae) from Zimbabwe. Journal of Zoology, 240(2), 221–231.
Galan, M., Pag`es, M., Cosson, J. F., & Kolokotronis, S. O. (2012). Next-generation sequencing for rodent barcoding: Species identification from fresh, degraded and environmental samples. PLoS One 7, e48374. https://doi.org/10.1371/journal.pone.0048374
Genelly, R. E. (1965). Ecology of the common mole-rat (Cryptomys hottentotus) in Rhodesia. Journal of Mammalogy, 46(4), 647–665. https://doi.org/10.2307/1377935
Gippoliti, S., Amori, G. (2011). A new species of mole-rat (Rodentia, Bathyergidae) from the Horn of Africa. Zootaxa, 2918(1), 39. https://doi.org/10.11646/zootaxa.2918.1.4
Gray, J. E. (1864). Notes on the species of sand-moles (Georychus). Proceedings of the Zoological Society of London, 1864, 123–125.
Greeff, J. M., & Bennett, N. C. (2000). Causes and consequences of incest avoidance in the cooperatively breeding mole-rat, Cryptomys darlingi (Bathyergidae). Ecology Letters, 3(4), 318–328. https://doi.org/10.1046/j.1461-0248.2000.00162.x
Happold, D. C. D. (2013). Mammals of Africa. Vol. 3. Rodents, hares and rabbits. Bloomsbury Publishing.
Hasegawa, M., Kishino, H., & Yano, T. A. (1985). Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22(2), 160–174. https://doi.org/10.1007/BF02101694
Herbst, M., & Bennett, N. C. (2001). Recrudescence of sexual activity in a colony of the Mashona mole-rat (Cryptomys darlingi): An apparent case of incest avoidance. Journal of Zoology, 254(2), 163–175. https://doi.org/10.1017/S095283690100067X
Hickman, G. C. (1978) Reactions of Cryptomys hottentotus to water (Rodentia: Bathyergidae). African Zoology, 13(2), 319-328. https://doi.org/10.1080/00445096.1978.11447632
Honeycutt, R. L. (2016). Family Bathyergidae. In D. E. Wilson, T. E. Lacher, R. A. Mittermeier (Eds.), Handbook of the mammals of the world. Vol.6: Lagomorphs and Rodents I, (pp. 352–370). Lynx Edicions.
Ingram, C. M., Burda, H., & Honeycutt, R. L. (2004). Molecular phylogenetics and taxonomy of the African mole-rats, genus Cryptomys and the new genus Coetomys Gray, 1864. Molecular Phylogenetics and Evolution, 31(3), 997–1014. https://doi.org/10.1016/j.ympev.2003.11.004
Zemanová, M., Šumbera, R., & Okrouhlík, J. (2012). Poikilothermic traits in Mashona mole-rat (Fukomys darlingi). Reality or myth? Journal of Thermal Biology, 37(7), 485–489. https://doi.org/10.1016/j.jtherbio.2012.04.001
Jerkovičová, D. (2010). The reproductive biology of the Mashona mole-rat (Fukomys darlingi) from southern Malawi. B. Sc. Thesis, 30pp, Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6), 587–589. https://doi.org/10.1038/nmeth.4285
Kiltie, R. A. (1982). Intraspecific variation in the mammalian gestation period. Journal of Mammalogy, 63(4), 646–652.
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16(2), 111–120. https://doi.org/10.1007/BF01731581
Krásová, J., Mikula, O., Bryja, J., Baptista, N. L., António, T., Aghová, T., & Šumbera, R. (2021). Biogeography of Angolan rodents: The first glimpse based on phylogenetic evidence. Diversity and Distributions, 27(12), 2571-2583. https://doi.org/10.1111/ddi.13435
Kock, D., Ingram, C. M., Frabotta, L. J., Honeycutt, R. L., & Burda, H. (2006). On the nomenclature of Bathyergidae and Fukomys n. gen. (Mammalia: Rodentia). Zootaxa, 1142(1), 51–55. https://doi.org/10.11646/zootaxa.1142.1.4
Mammal Diversity Database. (2022). Mammal Diversity Database (version 1.9.1). https://doi.org/10.52814/zenodo.4139818
Medina., A., Marti, D. A., & Bidau, C. J. (2007). Subterranean rodents of the genus Ctenomys (Caviomorpha, Ctenomyidae) follow the converse to Bergmann’s rule. Journal of Biogeography, 34(8), 1439–1454. https://doi.org/10.1111/j.1365-2699.2007.01708.x
Monadjem, A., Taylor, P. J., Denys, C., & Cotterill, F. P. D. (2015). Rodents of sub-Saharan Africa: A biogeographic and taxonomic synthesis. Walter de Gruyter GmbH & Co KG.
ORiain, M. J., Jarvis, J. U. M. (1998). The dynamics of growth in naked mole-rats: the effects of litter order and changes in social structure. Journal of Zoology, 246(1), 49-60.
Nguyen, L. T., Schmidt, H. A., von Haeseler, A., & Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32(1), 268–274. https://doi.org/10.1093/molbev/msu300
Patzenhauerová, H., Šklíba, J., Bryja, J., & Šumbera, R. (2013). Parentage analysis of Ansell’s mole-rat family groups indicates a high reproductive skew despite relatively relaxed ecological constraints on dispersal. Molecular Ecology, 22(19), 4988–5000. https://doi.org/10.1111/mec.12434
Peters, W. (1881). Über die von Herrn Major v. Mechow von seiner letzten Expedition nach Westafrika mitgebrachten Säugetiere. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin, 1881, 131–134.
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67(5), 901–904. https://doi.org/10.1093/sysbio/syy032
R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org
Roberts, A. (1946). Descriptions of numerous new subspecies of mammals. Annals of the Transvaal Museum, 20(4), 303–328.
Roberts, A. (1951). The mammals of South Africa. Trustees of “The Mammals of South Africa” Book Fund.
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61(3), 539–542. https://doi.org/10.1093/sysbio/sys029
Rstudio Team. (2020). Rstudio: Integrated development for R. Rstudio, PBC, Boston, MA. http://www.rstudio.com/
Scharff, A., Begall, S., Grütjen, O., & Burda, H. (1999). Reproductive characteristics and growth of Zambian giant mole-rats, Cryptomys mechowi (Rodentia: Bathyergidae). Mammalia, 63(2), 217–230. https://doi.org/10.1515/mamm.1999.63.2.217
Smithers, R. H. N., & Tello, J. L. P (1976). Check list and atlas of the mammals of Moçambique. Trustees of the National Museums and Monuments of Rhodesia, Salisbury, Rhodesia, Museum Memoir.
Šumbera, R., Burda, H. & Chitaukali, W.N. (2003). Reproductive biology of a solitary subterranean bathyergid rodent, the silvery mole-rat (Heliophobius argenteocinereus). Journal of Mammalogy, 84(1), 278-287.
Stamatakis, A. (2014). RaxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9), 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Tavaré, S. (1986). Some probabilistic and statistical problems in the analysis of DNA sequences. In R. M. Miura (Ed.), Lectures on mathematics in the life sciences. (Vol. 17, pp. 57–86). American Mathematical Society.
Thomas, O. (1897). Exhibition of small mammals collected by Mr. Alexander Whyte during his expedition to the Nyika plateau and Masuka Mountains, NR Nyasa. Proceedings of the Zoological Society of London, 1897, 430–436.
Thomas, O. (1895). On African mole-rats of the genera Georychus and Myoscalops. Annals and Magazine of Natural History, Series 6, 16(93), 238–241. https://doi.org/10.1080/00222939508680265
Thomas, O., & Wroughton, R.C. (1907). The Rudd exploration of South Africa: VIII: List of mammals obtained by Mr. Grant at Beira. Proceedings of the Zoological Society of London, 1907, 774-782.
Thomas, O. (1917). Notes on Georychus and its allies. Annals and Magazine of Natural History, Series 8, 20(120), 441–444. https://doi.org/10.1080/00222931709487034
Uhrová, M., Mikula, O., Bennett, N.C., Van Daele, P., Piálek, L., Bryja, J., Visser, J.H., Jansen van Vuuren, B., & Šumbera, R. (2022) Species limits and phylogeographic structure in two genera of solitary African mole-rats Georychus and Heliophobius. Molecular Phylogenetics and Evolution 167:107337.
Van Daele, P. A. A. G., Dammann, P., Meier, J. L., Kawalika, M., Van De Woestijne, C., & Burda, H. (2004). Chromosomal diversity in mole-rats of the genus Cryptomys (Rodentia: Bathyergidae) from the Zambezian region: With descriptions of new karyotypes. Journal of Zoology, 264(3), 317–326. https://doi.org/10.1017/S0952836904005825
Van Daele, P. A., Verheyen, E., Brunain, M., & Adriaens, D. (2007). Cytochrome b sequence analysis reveals differential molecular evolution in African mole-rats of the chromosomally hyperdiverse genus Fukomys (Bathyergidae, Rodentia) from the Zambezian region. Molecular Phylogenetics and Evolution, 45(1), 142–157. https://doi.org/10.1016/j.ympev.2007.04.008
Van Daele, P. A. A. G., Blonde, P., Stjernstedt, R., & Adriaens, D. (2013). A new species of African mole-rat (Fukomys, Bathyergidae, Rodentia) from the Zaire-Zambezi watershed. Zootaxa, 3636(1), 171–189. https://doi.org/10.11646/zootaxa.3636.1.7
Vejmělka, F., Okrouhlík, J., Lövy, M., Šaffa, G., Nevo, E., Bennett, N. C., & Šumbera, R. (2021). Heat dissipation in subterranean rodents: The role of body region and social organisation. Scientific Reports, 11, 2029. https://doi.org/10.1038/s41598-021-81404-3
Visser, J. H., Bennett, N. C., & Jansen van Vuuren, B., & Chiang, T. (2018). Spatial genetic diversity in the Cape mole-rat, Georychus capensis: Extreme isolation of populations in a subterranean environment. PloS One, 13, e0194165. https://doi.org/10.1371/journal.pone.0194165
Visser, J. H., Bennett, N. C., & Jansen van Vuuren, B. (2020). Phylogeny and biogeography of the African Bathyergidae: A review of patterns and processes. PeerJ, 7(10), e7730. https://doi.org/10.7717/peerj.7730
Wilson, D. E., & Reeder, D. M. (Eds.). (2005). Mammal species of the world: A taxonomic and geographic reference (Vol. 1). JHU press.
Wiedenová, P., Šumbera, R., & Okrouhlík, J. (2018). Social thermoregulation and socio-physiological effect in the subterranean Mashona mole-rat (Fukomys darlingi). Journal of Thermal Biology, 78, 367–373. https://doi.org/10.1016/j.jtherbio.2018.10.020
Yang, Z. (1994). Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. Journal of Molecular Evolution, 39(3), 306–314. https://doi.org/10.1007/BF00160154
Zelová, J., Šumbera, R., Sedláček, F., & Burda, H. (2007). Energetics in a solitary subterranean rodent, the silvery mole-rat, Heliophobius argenteocinereus and allometry of RMR in African mole-rats (Bathyergidae). Comparative Biochemistry and Physiology-Molecular and Integrative Physiology, 147, 412–419.
Acknowledgements
We would like to thank Femke Van Daele for assistance with tissue sampling, Enoki Sikaboa for assistance in collecting in Zambia, and Tim Lukas Flick for calculating Gompertz growth parameters. We are grateful to Radka Pešková for providing care for our animals. We thank to Ara Monadjem for providing us with presence records of F. darlingi published in Monadjem et al. (2015).
Funding
Open access publishing supported by the National Technical Library in Prague. This research was supported by the grant of the Czech Science Foundation (GAČR) n. 20-10222S, H2020 Synthesys + project (GB-TAF-8408), and Grant Agency of University of South Bohemia (GAJU) 014/2022/P.
Author information
Authors and Affiliations
Contributions
RS, HB, and PVD conceived and designed the study. RS provided funding. RS and WNC collected animals in the field. RS, DJ, KRC, and SB collected and analysed morphological, reproductive, and postnatal development data. RS, WNC, PVD, CGF, and NCB provided material for genetic analysis. CJ and SB performed karyotyping. MU performed genotyping. MU and OM performed phylogenetic analyses. RS, OM, KRC, SB, and MU wrote the first draft of the manuscript that was complemented by all authors. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This work complies with all current laws governing research in the Czech Republic, Germany, and Malawi. GRBC, the Technical Committee of the National Research Council of Malawi, permitted to carry out our research in Malawi and export the mole-rats.
Consent to participate
All authors gave consent to participate.
Consent for publication
All authors gave consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Šumbera, R., Uhrová, M., Begall, S. et al. The biology of an isolated Mashona mole-rat population from southern Malawi, with implications for the diversity and biogeography of the genus Fukomys. Org Divers Evol 23, 603–620 (2023). https://doi.org/10.1007/s13127-023-00604-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-023-00604-z