Abstract
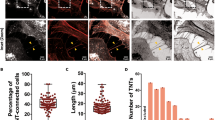
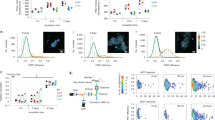
The interaction of α-synuclein with mitochondria in both typical and atypical Parkinson’s disease is a critical component of degeneration. The mechanism of cell-to-cell propagation of pathological α-synuclein in synucleinopathies is unclear. Intercellular exchange of mitochondria along tunnelling nanotubes has been described in other diseases, such as cancer; however, its role in synucleinopathies is unknown. Pathological α-synuclein species have been demonstrated previously to move from cell to cell via tunnelling nanotubes. This process was further explored using co-culture and monoculture systems to determine if α-synuclein binds to migrating mitochondria within tunnelling nanotubes. Super-resolution analysis via stimulated emission depletion microscopy showed interaction between α-synuclein with the mitochondrial outer membrane and the presence of alpha-synuclein associated with mitochondria in tunnelling nanotubes between 1321N1, differentiated THP-1 and SH-SY5Y cell types. siRNA knockdown of Miro1, a critical protein-bridging mitochondria to the motor adaptor complex, had no effect on mitochondrial density or α-synuclein association with mitochondria in tunnelling nanotubes. The results show that α-synuclein aggregates associate with mitochondria in intercellular tunnelling nanotubes, suggesting that mitochondria-mediated α-synuclein transfer between cells may contribute to cell-to-cell spread of α-synuclein aggregates and disease propagation.






Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- dH2O:
-
Deionised H2O
- DMEM:
-
Dulbecco’s modified Eagle medium
- FACS:
-
Fluorescence-activated cell sorting
- MSA:
-
Multiple system atrophy
- NGS:
-
Normal goat serum
- NHS:
-
Normal Horse serum
- PBS:
-
Phosphate-buffered saline
- PD:
-
Parkinson’s disease
- PMA:
-
Phorbol-12-myristate-13-acetate
- RIPA:
-
Radioimmunoprecipitation assay
- ROS:
-
Reactive oxygen species
- RT:
-
Room temperature
- SDS:
-
Sodium dodecyl sulfate
- siRNA:
-
Silencer RNA
- STED:
-
Stimulated emission depletion
- TnTs:
-
Tunnelling nanotubes
- TOM:
-
Translocase of outer membrane receptor
- WB:
-
Western blot
References
Abounit S, Delage E, Zurzolo C (2015) Identification and characterization of tunneling nanotubes for intercellular trafficking. Curr Protoc Cell Biol 67(1):12.10.1–12.10.21
Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, Olivo-Marin J, Melki R, Zurzolo C (2016a) Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J 35(19):2120–2138
Abounit S, Wu J, Duff K, Victoria G, Zurzolo C (2016b) Tunneling nanotubes: a possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 10(5):344–351
Agnati L, Fuxe K (2014) Extracellular-vesicle type of volume transmission and tunnelling-nanotube type of wiring transmission add a new dimension to brain neuro-glial networks. Philos Trans R Soc Lond B Biol Sci 369(1652):20130505–20130505
Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari B, Jha K, Barhanpurkar A, Wani M, Roy S, Mabalirajan U, Ghosh B, Agrawal A (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 9(33):994–1010
Austefjord M, Gerdes H, Wang X (2014) Tunneling nanotubes. Commun Integr Biol 7(1):e27934
Babenko V, Silachev D, Popkov B, Zorova L, Pevzner I, Plotnikov E, Sukhikh G, Zorov D (2018) Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 23(3):687
Bean B (2007) The action potential in mammalian central neurons. Nat Rev Neurosci 8(6):451–465
Bowdish D (2011) Maintenance & culture of THP-1 cells. [online] Hamilton: Bowdish lab macrophage biology, pp.1-2. Available at: http://www.bowdish.ca/lab/wp-content/uploads/2011/07/THP-1-propagation-culture.pdf [Accessed June 2018]
Brickley K, Stephenson F (2011) Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J Biol Chem 286(20):18079–18092
Calì T, Ottolini D, Negro A, Brini M (2012) α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem 287(22):17914–17929
Chinnery H, Pearlman E, McMenamin P (2008) Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol 180(9):5779–5783
Chinta S, Mallajosyula J, Rane A, Andersen J (2010) Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 486(3):235–239
Chu Y, Morfini G, Langhamer L, He Y, Brady S, Kordower J (2012) Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 135(7):2058–2073
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441(7097):1162–1166
Costanzo M, Abounit S, Marzo L, Danckaert A, Chamoun Z, Roux P, Zurzolo C (2013) Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J Cell Sci 126(16):3678–3685
David G, Barrett E (2003) Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol 548(2):425–438
Di Maio R, Barrett P, Hoffman E, Barrett C, Zharikov A, Borah A, Hu X, McCoy J, Chu C, Burton E, Hastings T, Greenamyre J (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8(342):342ra78–342ra78
Dieriks B, Park T, Fourie C, Faull R, Dragunow M, Curtis M (2017) α-Synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson’s disease patients. Sci Rep 7:42984
Dilsizoglu Senol A, Pepe A, Grudina C, Sassoon N, Reiko U, Bousset L, Melki R, Piel J, Gugger M, Zurzolo C (2019) Effect of tolytoxin on tunneling nanotube formation and function. Sci Rep 9(5741):5741
Dong L, Kovarova J, Bajzikova M, Bezawork-Geleta A, Svec D, Endaya B, Sachaphibulkij K, Coelho A, Sebkova N, Ruzickova A, Tan A, Kluckova K, Judasova K, Zamecnikova K, Rychtarcikova Z, Gopalan V, Andera L, Sobol M, Yan B, Pattnaik B, Bhatraju N, Truksa J, Stopka P, Hozak P, Lam A, Sedlacek R, Oliveira P, Kubista M, Agrawal A, Dvorakova-Hortova K, Rohlena J, Berridge M, Neuzil J (2017) Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. eLife, 6(e22187)
Dupont M, Souriant S, Lugo-Villarino G, Maridonneau-Parini I, Vérollet C (2018) Tunneling nanotubes: intimate communication between myeloid cells. Front Immunol, 9(43)
Eugenin E, Gaskill P, Berman J (2009) Tunneling nanotubes (TNT). Commun Integr Biol 2(3):243–244
Fiebig C, Keiner S, Ebert B, Schäffner I, Jagasia R, Lie D, Beckervordersandforth R (2019) Mitochondrial dysfunction in astrocytes impairs the generation of reactive astrocytes and enhances neuronal cell death in the cortex upon photothrombotic lesion. Front Mol Neurosci, 12
Follett J, Darlow B, Wong M, Goodwin J, Pountney D (2013) Potassium depolarization and raised calcium induces α-synuclein aggregates. Neurotox Res 23(4):378–392
Follett J, Bugarcic A, Yang Z, Ariotti N, Norwood SJ, Collins BM, Parton RG, Teasdale RD (2016) Parkinson Diseaselinked Vps35 R524W Mutation Impairs the Endosomal Association of Retromer and Induces α-Synuclein Aggregation. J Biol Chem 291(35):18283–18298
Fransson Å, Ruusala A, Aspenström P (2006) The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344(2):500–510
Goodwin J, Nath S, Engelborghs Y, Pountney DL (2013) Raised calcium and oxidative stress cooperatively promote alpha-synuclein aggregate formation. Neurochem Int 62(5):703–711
Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman D, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin J, Männel D, Zurzolo C (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 11(3):328–336
Grassi D, Howard S, Zhou M, Diaz-Perez N, Urban N, Guerrero-Given D, Kamasawa N, Volpicelli-Daley L, LoGrasso P, Lasmézas C (2018) Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc Natl Acad Sci 115(11):E2634–E2643
Henrichs V, Grycova L, Barinka C, Nahacka Z, Neuzil J, Diez S, Rohlena J, Braun M, Lansky Z (2020) Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments. Nat Commun 11(1):3123
Ježek J, Cooper K, Strich R (2018) Reactive oxygen species and mitochondrial dynamics: the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants 7(1):13
Kann O, Kovács R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292(2):C641–C657
Kay L, Pienaar I, Cooray R, Black G, Soundararajan M (2018) Understanding Miro GTPases: implications in the treatment of neurodegenerative disorders. Mol Neurobiol 55(9):7352–7365
Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira A, Manova-Todorova K, Moore M (2012) Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One 7(3):e33093
MacAskill A, Rinholm J, Twelvetrees A, Arancibia-Carcamo I, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler J (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61(4):541–555
Melo TQ, Van Zomeren KC, Ferrari MF, Boddeke HW, Copray JC (2017) Impairment of mitochondria dynamics by human A53T alpha-synuclein and rescue by NAP (davunetide) in a cell model for Parkinson's disease. Exp Brain Res 235(3):731–742
Katoh M, Wu B, Nguyen HB, Thai TQ, Yamasaki R, Lu H, Rietsch AM, Zorlu MM, Shinozaki Y, Saitoh Y, Saitoh S, Sakoh T, Ikenaka K, Koizumi S, Ransohoff RM, Ohno N (2017) Polymorphic regulation of mitochondrial fission and fusion modifies phenotypes of microglia in neuroinflammation. Sci Rep 7(1):4942
Nakahira K, Haspel J, Rathinam V, Lee S, Dolinay T, Lam H, Englert J, Rabinovitch M, Cernadas M, Kim H, Fitzgerald K, Ryter S, Choi A (2010) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12(3):222–230
O'Donnell KC, Lulla A, Stahl MC, Wheat ND, Bronstein JM, Sagasti A (2014) Axon degeneration and PGC-1alpha-mediated protection in a zebrafish model of alpha-synuclein toxicity. Dis Model Mech 7(5):571–582
Okafo G, Prevedel L, Eugenin E (2017) Tunneling nanotubes (TNT) mediate long-range gap junctional communication: implications for HIV cell to cell spread. Sci Rep 7(1):16660
Panasiuk M, Rychłowski M, Derewońko N, Bieńkowska-Szewczyk K (2018) Tunneling nanotubes as a novel route of cell-to-cell spread of herpesviruses. J Virol, 92(10)
Parihar M, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65(7–8):1272–1284
Poliak S, Peles E (2003) The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci 4(12):968–980
Prots I, Veber V, Brey S, Campioni S, Buder K, Riek R, Bohm KJ, Winner B (2013) Alpha-Synuclein oligomers impair neuronal microtubule-kinesin interplay. J Biol Chem 288(30):21742–21754
Prots I, Grosch J, Brazdis R, Simmnacher K, Veber V, Havlicek S, Hannappel C, Krach F, Krumbiegel M, Schütz O, Reis A, Wrasidlo W, Galasko D, Groemer T, Masliah E, Schlötzer-Schrehardt U, Xiang W, Winkler J, Winner B (2018) α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc Natl Acad Sci 115(30):7813–7818
Prusiner S, Woerman A, Mordes D, Watts J, Rampersaud R, Berry D, Patel S, Oehler A, Lowe J, Kravitz S, Geschwind D, Glidden D, Halliday G, Middleton L, Gentleman S, Grinberg L, Giles K (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci 112(38):E5308–E5317
Randall T, Moores C, Stephenson F (2013) Delineation of the TRAK binding regions of the kinesin-1 motor proteins. FEBS Lett 587(23):3763–3769
Rcom-H’cheo-Gauthier AN, Meedeniya ACB, Pountney DL (2017) Calcipotriol inhibits α-synuclein aggregation in SH-SY5Y neuroblastoma cells by a Calbindin-D28k-dependent mechanism. J Neurochem 141(2):263–274
Reeve A, Ludtmann M, Angelova P, Simcox E, Horrocks M, Klenerman D, Gandhi S, Turnbull D, Abramov A (2015) Aggregated α-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis 6(7):e1820–e1820
Reyes J, Sackmann C, Hoffmann A, Svenningsson P, Winkler J, Ingelsson M, Hallbeck M (2019) Binding of α-synuclein oligomers to Cx32 facilitates protein uptake and transfer in neurons and oligodendrocytes. Acta Neuropathol 138(1):23–47
Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark G, Ingelsson M, Bergström J, Roybon L, Erlandsson A (2017) Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J Neurosci 37(49):11835–11853
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes H (2004) Nanotubular highways for intercellular organelle transport. Science 303(5660):1007–1010
Ryan B, Hoek S, Fon E, Wade-Martins R (2015) Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem Sci 40(4):200–210
Sartori-Rupp A, Cordero Cervantes D, Pepe A, Gousset K, Delage E, Corroyer-Dulmont S, Schmitt C, Krijnse-Locker J, Zurzolo C (2019) Correlative cryo-electron microscopy reveals the structure of TNTs in neuronal cells. Nat Commun 10(342):342
Shaltouki A, Hsieh C, Kim M, Wang X (2018) Alpha-synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson’s models. Acta Neuropathol 136(4):607–620
Shen J, Zhang J, Xiao H, Wu J, He K, Lv Z, Li Z, Xu M, Zhang Y (2018) Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis, 9(2)
Smith I, Shuai J, Parker I (2011) Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J 100(8):L37–L39
Tardivel M, Bégard S, Bousset L, Dujardin S, Coens A, Melki R, Buée L, Colin M (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun 4(117):117
Valdinocci D, Radford RA, Siow SM, Chung RS, Pountney DL (2017) Potential modes of intercellular α-synuclein transmission. Int J Mol Sci. 18(2)
Valdinocci D, Grant GD, Dickson TC, Pountney DL (2018) Epothilone D inhibits microglia-mediated spread of alpha-synuclein aggregates. Mol Cell Neurosci 89:80–94
Valdinocci D, Simões RF, Kovarova J, Cunha-Oliveira T, Neuzil J, Pountney DL (2019) Intracellular and intercellular mitochondrial dynamics in Parkinson’s disease. Front Neurosci 13:930
van Spronsen M, Mikhaylova M, Lipka J, Schlager M, van den Heuvel D, Kuijpers M, Wulf P, Keijzer N, Demmers J, Kapitein L, Jaarsma D, Gerritsen H, Akhmanova A, Hoogenraad C (2013) TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77(3):485–502
Vijayakumaran S, Nakamura Y, Henley JM, Pountney DL (2019) Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Mol Cell Neurosci 101:103416
Wang X, Gerdes H (2015) Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ 22(7):1181–1191
Wang X, Schwarz T (2009) The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136(1):163–174
Wang X, Becker K, Levine N, Zhang M, Lieberman A, Moore D, Ma J (2019) Pathogenic alpha-synuclein aggregates preferentially bind to mitochondria and affect cellular respiration. Acta Neuropathol Commun 7(1):41
Watkins SC, Salter RD (2005) Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules. Immunity 23(3):309–318
Xie W, Chung KK (2012) Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J Neurochem 122(2):404–414
Zhou R, Yazdi A, Menu P, Tschopp J (2010) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225
Acknowledgements
We gratefully acknowledge Marie Olšinová and the Imaging Methods Core Facility at BIOCEV, institution supported by the MEYS CR (Large RI Project LM2018129 Czech-Bioimaging) and ERDF (project no. CZ.02.1.01/0.0/0.0/16_013/0001775) for their support with obtaining imaging data presented in this paper.
Funding
This study is financially supported by Griffith University and BIOCEV. JN was supported in part by grant 20-05942S from the Czech Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valdinocci, D., Kovarova, J., Neuzil, J. et al. Alpha-Synuclein Aggregates Associated with Mitochondria in Tunnelling Nanotubes. Neurotox Res 39, 429–443 (2021). https://doi.org/10.1007/s12640-020-00285-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00285-y