Abstract
Purpose
The aim of this study was the comparative analysis of the parasite communities of new populations of invasive pumpkinseed (Lepomis gibbosus) in western Ukraine with pumpkinseed from Czechia, where populations have rapidly expanded over the last two decades.
Methods
Sampling took place at three localities in the western part of Ukraine (i.e. Dobrotvir Reservoir (Vistula basin), Burshtyn Reservoir (Dniester basin), Mynai Pond (Danube basin)) and four in Czechia (i.e. Oxbow D2, Heršpický Pond (Danube basin), and Kolín oxbow and Římov Reservoir (Elbe basin).
Results
In total, 11 parasite taxa were recorded in Ukraine and 17 in Czechia. Four species were co-introduced from North America with their host, i.e. the myxosporean Myxobolus dechtiari, the monogeneans Onchocleidus dispar and Onchocleidus similis, and metacercariae of a trematode Posthodiplostomum centrarchi. High dominance indices were related to a high abundance of co-introduced parasites, i.e. O. similis in Mynai pond and P. centrarchi in Dobrotvir Reservoir. Overall abundance of acquired parasites was generally low.
Conclusion
This study shows that parasite communities in recently established pumpkinseed populations in the western part of Ukraine and Czechia are less diverse than those established in Europe for decades. The generally low parasite load in these new populations may play an important role in their ability to successfully establish and create strong populations by providing a competitive advantage over local species.
Similar content being viewed by others
Introduction
Parasites and pathogens are important components of natural ecosystems; their absence or presence affects interactions between a whole range of species in the community [1]. An increasing problem worldwide, however, is the presence of non-native parasites, which can negatively affect biota within their newly invaded ecosystems [2]. Many of these parasites are co-introduced together with their natural hosts [3, 4]. These parasites are mainly specific to their non-native hosts and remain part of their parasite fauna and do not infect local species during the host’s range expansion [5,6,7]. In some cases, however, the non-native host species may distribute new, non-native parasites, that successfully infect local hosts [8,9,10]. In such cases, the introduced non-native species may have a negative influence on local fauna as the new hosts lack the protective immunity that native hosts acquired during their shared coevolution history [11, 12].
Spread of parasites, pathogens and diseases is commonly associated with non-native fishes, and this can have serious consequences for local species as native hosts and non-native parasites have had no time to evolve an interaction balance [9, 13]. On the other hand, while local parasites can quickly assimilate new hosts, the temporary escape of parasites in the early stages of invasion allows non-native species to adapt to new environments and establish stable populations [14]. Subsequently, parasite infections of non-native host populations tend to increase over time after the onset of invasion, though infection rates rarely reach quantitative values similar to those of native species [15].
The pumpkinseed sunfish (Lepomis gibbosus L., 1758; Actinopterygii: Centrarchidae) is a North American freshwater species that was introduced into Europe at the end of the nineteenth century (1877) as an ornamental fish for garden ponds [16,17,18]. It is now distributed in at least 28 European states and in the Asian part of Turkey [19,20,21,22] and was included in the list of Invasive Species of Union Concern in 2019 [23]. In Ukraine, pumpkinseed was first recorded in 1918 in the Lower Danube [24], since when the fish has become common in the rivers and lakes of south Ukraine, the Dnipro (Dnieper) basin up to Kyiv, in small rivers of the Azov coast and in coastal waters of the Black Sea and the Sea of Azov [21, 25, 26]. In Czechia, the species first occurred in 1929 in the Třeboň region, where it was accidentally introduced with carp (Cyprinus carpio L., 1758) fry from the former Yugoslavia [27]. During the following years, just a few isolated populations were established [28]; however, rapid expansion into a wide range of water bodies has been observed over recent decades.
While there has been a relative scarcity of information available on parasites of pumpkinseed from its non-native range over the twentieth century (see e.g. [29, 30], and references in [31] for exceptions), research interest in this topic has increased over the last two decades. This is due, in part, to new data on co-introduced species such as myxozoans [32], monogeneans (e.g. [6, 33,34,35]), cestodes [36] and trematodes [37, 38]. In addition, several studies have now documented, or summarised, original data on parasite communities for populations from Western, Central and Eastern Europe. For example, pumpkinseed parasites are now well documented from Bulgaria [39], England [40], France [33, 36, 41, 42], Germany [43], Moldova [44, 45], Poland [30, 46], Slovakia and Serbia [31, 47]. However, despite the recent rapid expansion of pumpkinseed in both Czechia and Ukraine, data on their parasites are only known from a relatively small area of their host distribution. In Ukraine, pumpkinseed parasites have been studied in the Dnieper basin, Lower Danube and brackish water estuaries [29, 34, 48], while data from the western part of the country remains absent. Data from Czechia includes studies limited to a single parasite taxon, i.e. monogeneans, ciliates and crustaceans (e.g. [6, 49,50,51], though parasite community data have recently been published for a few isolated sites in the Danube and Elbe basins [42]. Thus, the aim of this study was to present data on the parasite communities of new pumpkinseed populations in the western part of Ukraine, and to these with data from Czechia, where pumpkinseed have rapidly expanded their distribution over the last two decades.
Material and Methods
Study Localities
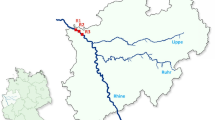
Sampling took place at three localities in the western part of Ukraine over June to September 2022, i.e. (1) Dobrotvir Reservoir (50.159799 N, 24.401295 E; Vistula basin); (2) Burshtyn Reservoir (49.257926 N, 24.639904 E; Dniester basin); and (3) Mynai Pond, Latorica River (48.58683N, 22.29009 E; Danube basin) (Fig. 1). In Czechia, fish were sampled from four waterbodies between 2015 and 2021, i.e. (4) oxbow D2 of the Dyje River (48.672910 N, 16.923282 E; Danube basin, 2015); (5) Heršpický Pond (49.114706 N, 16.932734 E; Danube basin, 2021); (6) Kolín oxbow (50.020453 N, 15.228106 E; Elbe basin, 2020); (7) Římov Reservoir, Malše River (48.835597 N, 14.482397 E; Elbe basin, 2018) (Fig. 1). The first locality, Dobrotvir Reservoir, is the only site in the Baltic basin and acts as a cooling reservoir for the Dobrotvir Power Plant on the Bug River (Vistula River tributary). The other two Ukrainian localities belong to the Black Sea basin. The Burshtyn Reservoir was built in 1965 on the Hnyla Lypa River (Dniester River tributary) as a cooling reservoir for the Burshtyn power plant but has now been reclassified as a natural protected area [52, 53]. The pond in the village of Mynai in the Transcarpathian region of Ukraine is a small fire pond located within the settlement and lacks any connection with a natural water body, though it lies within the Latorica River basin (Tisza River tributary, Danube River basin). In Czechia, both Oxbow D2 and Heršpický Pond lie within the Danube River basin. The first of these is an old oxbow in the lowest section of the Dyje River (Morava tributary) floodplain, created after the channelisation of the Dyje during the 1970s. Surrounded by trees and with a sand/mud bottom, almost 20% of the oxbow’s area is covered with aquatic vegetation. Pumpkinseed invaded Oxbow D2 sometime after 2010, since when they have established a large population [49]. Heršpický pond lies in the Svratka River basin and is now protected due to the occurrence and reproduction of several protected amphibian species. Pumpkinseed were accidentally introduced into the pond sometime around 2019, after which they quickly established a stable population (Zajíček R., personal observation). The final two Czech localities lie in the Elbe River basin (North Sea drainage). The Kolín oxbow was created after the Elbe River was channelised between 1913 and 1927 and is now recognised as a natural monument (Kolínské tůně) for protecting local ecosystems of the Elbe floodplain and their typical flora and fauna. Pumpkinseed was accidentally introduced into the oxbow sometime around the beginning of the twenty-first century. The canyon-shaped Římov Reservoir was constructed in 1978 by damming the River Malše (Vltava River tributary) for water supply and flood control. It is a 12 km long mesotrophic to eutrophic reservoir that lacks submerged aquatic macrophytes in the littoral zone due to its steep banks and water level fluctuations [54]. Occurrence of pumpkinseed in the reservoir has been known since 2008, the species probably entering the reservoir from the Malše River, into which it was introduced from adjacent fishponds (Kubečka J., personal observation).
Schematic map with the study areas indicated. See “Material and methods” for the locality numbers
Fish sampling and Processing
All fish were collected using an angular 4 mm mesh trap-net (tetrahedron, 0.8 m edge), a 7 m, 1 cm mesh seine net or through backpack electrofishing (Lena, Bednář, Czech Republic). The fish were transported alive in aerated containers with water from the sampling locality to the laboratory, where they were subsequently held in aerated aquaria. Parasitological examinations were performed within 24 h after sampling, according to Kvach et al. [55].
A total of 127 fish were sampled and examined, comprising 52 specimens from Ukraine and 75 from Czechia (Table 1), with 22 fish collected from the Baltic basin, 70 from the Black Sea basin (12 in the Dniester and 58 in the Danube River drainages), and 32 from the North Sea basin. The standard (SL, mm) and total (TL, mm) lengths of each fish were measured before sectioning.
Parasite Analysis
Fish tissues and organs were examined for the presence of parasites using Konus Crystal 7x-45x (Konus Optical and Sport Systems, Italy) and Olympus SZX 10 (Olympus Optical Co., Tokyo, Japan) stereomicroscopes. The fins, skin and gills were all observed in a Petri dish with water from the sampling locality, while the eyes, muscles and internal organs were examined after compression between two 9 × 13-cm glass plates. Microparasites were studied alive, monogeneans were preserved in GAP (Glycerine-ammonium-picrate) and prepared as semi-permanent slides [56], and digeneans, cestodes and nematodes preserved in hot 4% formaldehyde and stained with iron acetic carmine, dehydrated in ethanol of increasing concentration and mounted in Canada balsam as permanent slides [57, 58]. Acanthocephalans were preserved in 70% ethanol, pressed between two glass slides and then mounted in glycerol as temporary slides for light microscopy, while glochidia and crustaceans were preserved in 4% formaldehyde. All parasites were identified to species level where possible, or to the lowest possible taxon, under an Olympus IX 83 light microscope (Olympus Optical Co., Tokyo, Japan). Images of Posthodiplostomum centrarchi Hoffman, 1958 were obtained using an Olympus BX53 light microscope (Olympus Optical Co., Tokyo, Japan) fitted with the Olympus cellSens Standard v.3.2 digital image analysis package (Olympus Optical Co., Hamburg, Germany).
Indices of prevalence (P, %), mean intensity (MI), intensity range (IR) and mean abundance (A) were calculated for each parasite species [59], with standard deviation (sd) calculated for mean parameters. Similarity between parasite communities at different localities was evaluated using the Czekanowski-Sørensen Index (CSI) [60]. Differences in parasite abundance and infracommunity richness (i.e. number of parasite species per host) [59] between different pumpkinseed populations were tested using the Kruskal–Wallis ANOVA function in Statistica v.14.0.0.15 (TIBCO Software Inc., USA). Shannon Wiener, Dominance and Equitability diversity indices were calculated using PAST software (PAlaeontologicalSTatistics, v.1.77, http://folk.uio.no/ohammer/past/) [61] for populations with two or more parasite taxa. Only metazoan parasites were used for diversity indices as the intensity of infection was not possible to calculate for protozoan parasites. The same software was also used for permutation tests to compare diversity indices between the same locality. Bonferroni correction for multiple comparisons was used, with the level of significance set at p < 0.01.
Molecular Analysis
Parasite specimens were preserved in 96% ethanol for the identification of white grub metacercariae from internal organs. DNA was extracted from each metacercaria using the innuPREP Forensic Kit (Analytik Jena, Germany). PCR was undertaken using the internal transcribed spacer 1 (ITS1) specific primers BD1 (forward: 5′-GTCGTAACAAGGTTTCCGTA-3′) and 4S (reverse: 5′-TCTAGATGCGTTCGAARTGTCGATG-3′) [62, 63]. The PCR reaction mix had a total volume of 10 μl, comprising 4 μl of extracted DNA, 0.3 μl primer, 2 μl buffer A, 0.2 μl dNTPs, 0.2 μl MgCl2, 0.1 μl Taq polymerase and 2.9 μl ddH2O. The KAPA2G Robust Hot-Star PCR Kit (Kapabiosystems, USA) was used for PCR analysis. Amplification was undertaken using a Mastercycler ep gradient S thermocycler (Eppendorf, Germany) with an annealing temperature of 57 °C. The PCR product was then purified using an ExoSAP-IT kit (Affymetrix Inc., Santa Clara, USA), according to the manufacturer’s protocol. The PCR products were then checked on 1.5% agarose gel with GoodView™ Nucleic Acid Stain. Sanger sequencing of the PCR products was performed commercially at GATC Biotech (Germany) and the sequences were edited and aligned using Geneious v.9.0.5 software [64].
The obtained sequences were compared with the NCBI database using BLASTn to assess sequence similarity with Posthodiplostomum sp. 3 (GenBank accession no. HM064955), Posthodiplostomum sp. 5 (HM064958) and Posthodiplostomum sp. 8 (HM064962) [65], and with Posthodiplostomum cf. minimum centrarchi (KX931441, MF170981-82, MF170992-93) [37].
Results
Pumpkinseed Parasite Community Composition
In total, 25 parasite taxa were recorded on pumpkinseed from the two study regions (11 taxa in Ukraine, and 17 in Czechia) (Table 2). In Ukraine, the richest parasite community, consisting of seven taxa, occurred in the Dobrotvir Reservoir. Only one species was found in the Burshtyn Reservoir and four in the Mynai Pond. In Czechia, the richest parasite community was found in the Kolín and D2 oxbows (nine and seven taxa, respectively; Table 3). Most of the localities compared had no common parasite species. In the other cases, similarity was less than 50%, being 25% between the Burshtyn and Dobrotvir reservoirs, 23.5% between the D2 and Kolín oxbows, 22.2% between D2 and Římov, 16.7% between Kolín and Římov and 14.3% between D2 and Dobrotvir. Only two species occurred in both Ukraine and Czechia, the North American monogenean Onchocleidus dispar Mueller, 1936 and the trematode metecarcariae P. centrarchi (ICS = 14.3%).
We found four species co-introduced from North America along with their host, the myxosporean Myxobolus dechtiari Cone & Anderson, 1977 (Mynai pond, Burshtyn Reservoir), the monogeneans O. dispar (Mynai pond, Oxbow D2) and Onchocleidus similis Mueller, 1936 (Mynai pond) and metacercariae of the trematode P. centrarchi (Dobrotvir Reservoir, Oxbow D2). Identification of P. centrarchi was confirmed through genetic analysis, with two sequences obtained from the Dobrotvir Reservoir and one from Oxbow D2. One of the sequences from the Dobrotvir Reservoir and the D2 oxbow were identical and differed from the second sequence from the Dobrotvir Reservoir with one substitution and one gap. All sequences showed 99.9% correspondence with P. centrarchi from pumpkinseed collected in the Hudson River in Canada (GeneBank Acc. No. MH521251), Hungary (MN080282) and in Portugal (MF171006; denoted as P. cf. minimum) Morphological characteristics were—body length: 1467.4 ± 190.0 (1171.4–1673.6), forebody length: 996.6 ± 93.2 (855.2–1154.59) and width: 495.2 ± 79.5 (377.4–617.4), hindbody length 536.9 ± 75.3 (406.4–635.1) and width 476.5 ± 93.6 (350.9–630.2), oral sucker length 45.5 ± 5.7 (36.9–55.6) and width 47.3 ± 5.8 (41.2–57.9), ventral sucker length 56.6 ± 7.7 (45.1–70.1) and width 55.6 ± 5.3 (49.8–63.9) and Brandes organ length 166.9 ± 20.0 (136.1–196.2) and width 164.9 ± 33.9 (121.5–229.9) (Fig. 2). Where present, the North-American parasites reached highest prevalence and abundance, representing 89% of all parasites collected (excluding ciliates, for which abundance was not calculated; Fig. 3A). No co-introduced North-American parasites were observed in the Burshtyn Reservoir (Ukraine), Heršpický Pond, Kolín oxbow or in Římov Reservoir (Czechia) (Table 2, Fig. 3).
A Parasite abundance (without microparasites), and B infracommunity species richness in Lepomis gibbosus collected from different localities in Ukraine and the Czech Republic (bars = mean, whiskers = S.E.). Grey bars = values for all parasite taxa, within which the hatched parts represent parasites acquired in Europe
Regarding parasites acquired in Europe, all except two taxa each occurred at one locality only, with generally low abundance (Fig. 3A). The branchiuran Argulus foliaceus (Linnaeus, 1758) was the most frequent species found at the three localities in Czechia, though all infected fish were parasitised with a single specimen only. Trichodinid ectoparasites were observed at two localities in Czechia, with 56% of fish infected at the Kolín oxbow and just one fish infected at Oxbow D (Table 2). In addition to local species, pumpkinseed collected in Czechia acquired three Asian species, glochidia of invasive Chinese pond mussels (Sinanodonta woodiana (I. Lea, 1834)) in Oxbow D2, and ergasilid copepods Neoergasilus japonicus Harada, 1930 and larvae of the eel (Anguilla anguilla (L., 1758)) nematode Anguillicola crassus Kuwahara, Niimi & Itagaki, 1974 at the Kolín oxbow. In comparison, parasite acquisition in Ukrainian regions was rare, with most parasites found being of North American origin (see above).
Comparative Analysis of Parasite Abundance
Parasite abundance differed significantly between localities (Kruskal–Wallis ANOVA; H (6, N = 127) = 66.7, p < 0.0001; Fig. 3A), with just one or two species recorded at three localities (Burshtyn, Heršpický pond and Římov Reservoir) and a mean abundance of greater than five at three others (Mynai, Dobrotvir and Oxbow D2; Table 2). Interestingly, these latter three sites were the only ones where co-introduced North American parasites were found. Regarding parasites acquired in Europe, i.e. excluding North American parasites, differences in parasite abundance between localities diminished and, while overall analysis showed significant differences (H (6, N = 127) = 34.9, p < 0.0001), post-hoc comparison tests indicated no significant difference between localities after correction for multiple comparisons.
Parasite Diversity
Diversity varied greatly among sites, both in Czechia and Ukraine (Table 3). Infracommunity species richness was highest in the Kolín and D2 oxbows, and lowest in the Burshtyn and Heršpický ponds (Fig. 3B). Component community species richness ranged from one in both regions (Burshtyn, Heršpický pond) to seven in Ukraine (Dobrotvir) and nine in Czechia (Kolín). Analysis of localities with two or more parasite taxa indicated that Ukrainian localities were characterised by a low Shannon diversity index and equitability, alongside high dominance, this being related to a high abundance of co-introduced parasites, i.e. the monogenean O. similis in the Mynai pond and the trematode P. centrarchi in the Dobrotvir Reservoir (Tables 2, 3). On the other hand, we observed a high Shannon index, but rather low dominance and equitability at Czech localities (Table 3). The maximum Shannon index, recorded at the Kolín oxbow (1.78), was significantly higher than that at Mynai, Dobrotvir, Římov and Oxbow D2 (permutation test, p < 0.001). In addition, Shannon diversity at Mynai was significantly lower than that at Oxbow D2 (p < 0.001). Dominance indices at Mynai and Dobrotvir were significantly higher than those at all other Czech localities (all p ≤ 0.002), while dominance at Kolín was lower than that at Oxbow D2 and Římov. Equitability was significantly higher at Czech localities compared with Ukrainian localities and was also significantly lower at Oxbow D2 compared with other Czech localities (p < 0.001 for all comparisons).
Discussion
We compared the parasite communities of new invasive pumpkinseed populations in the western part of Ukraine with pumpkinseed from Czechia, where populations have rapidly expanded over the last two decades. In total, 11 parasite taxa were recorded in Ukraine and 17 in Czechia, with four species having been co-introduced from North America with their host. Metacercariae of P. centrarchi were registered in pumpkinseed from Ukraine for the first time, and we also provide the first report of the Posthodiplostomum sp. 3 lineage sensu Locke et al. [65]. Previously, P. centrarchi metacercariae had been found in largemouth bass Micropterus salmoides (Lacépède, 1802) in Ukraine, having been transported from France and held in aquacultural ponds in Kyiv [35]. These trematodes are related to the Posthodiplostomum sp. 8 lineage, which appears to be specific to largemouth bass [35, 65]. Over the last decade, metacercariae of P. centrarchi have been recorded in several European countries, including Bulgaria, Czechia, France, Germany, Hungary, Poland, Portugal and Slovakia [37, 38, 42, 43, 46, 66], with most belonging to the Posthodiplostomum sp. 3 lineage collected from pumpkinseed. Though a recent study of pumpkinseed populations in southern Ukraine failed to confirm the presence of this parasite species in the region [48], further expansion of P. centrarchi to the east is to be expected. The definitive hosts of this parasite are herons (Ardeidae) [38, 67], which spread the eggs of the parasites to new localities. The presence of established populations of the second intermediate host in Ukraine, i.e. the pumpkinseed, makes possible the completion of the parasite’s life cycle.
Among the other co-introduced parasites, the myxozoan M. dechtiari has already been recorded in Ukraine in Lake Kartal and the brackish Khadzhibey Estuary [48]. This parasite is currently known in invasive pumpkinseed in Hungary, Moldova and Portugal [32, 44]. Considering the recent finding of this parasite in the Tisza River basin in Ukraine, close to the Hungarian border, and in the Lower Danube basin (Lake Kartal), it is plausible that the range of this species is much wider in the Danubian basin.
Two co-introduced monogenean species, O. dispar and O. similis, are widely distributed in Europe, having been recorded in non-native pumpkinseed populations in Austria, Bulgaria, Croatia, Czechia, France, Germany, Italy, Norway, Serbia and Slovakia [6, 33, 42, 43, 47, 68, 69]. In Ukraine, both species are known from the Danube basin [70], but only O. dispar has been found in the Dnipro (Dnieper) river basin [34, 48]. In Czechia, while both Onchocleidus species have previously been recorded from pumpkinseed caught in the Dyje River in the Danube River basin and the Donbas sandpit in the Morava River basin [6, 42], recent pumpkinseed populations in waterbodies of the Dyje River floodplain appear to be parasitised with O. dispar only [this study, 42]. These results suggest that the pumpkinseed making up the most recent invasion of lentic waterbodies of the Dyje River floodplain do not originate from long-established Dyje pumpkinseed populations, which are parasitised with both O. similis and O. dispar [6]. This study also confirmed the presence of O. dispar and O. similis in the Latorica river basin in the Transcarpathian region of Ukraine. Pumpkinseed were first recorded in the Tisza basin in the 2000s, since when the species has started to establish large populations in many adjacent waterbodies [71, 72]. The presence of these non-native monogeneans strongly suggests that these populations are related to the old Danubian pumpkinseed populations.
The low parasite load observed in most of the localities in Czechia and the western part of Ukraine examined in this study could be explained by the young age of the pumpkinseed populations. Low parasite prevalence was particularly apparent in relation to species acquired in Europe. The occurrence of pumpkinseed in the Bug River basin (Dobrotvir Reservoir) is new, and our finding is, in fact, the first record of this fish species in the Vistula River basin, the species only having been recorded in the Oder River basin in the Baltic Sea catchment prior to this study [73, 74]. Despite being a recent population, pumpkinseed parasite species richness in the Dobrotvir Reservoir was relatively high, reaching the same level as that found in other Ukrainian localities [48]. However, most parasite species found only occurred accidentally, with the North American trematodes P. centrarchi predominating, resulting in the high dominance index recorded at this locality. The same situation was observed at Oxbow D2 on the Dyje River in Czechia, where the population was approximately three to four years old. Fish here had acquired seven parasite taxa, though at very low abundance and prevalence, with P. centrarchi representing the majority of parasites in the community.
Extreme parasite avoidance was observed at Burshtyn Reservoir and Heršpický Pond, where only one and two parasites were found, respectively. The Burshtyn Reservoir (Upper Dniester River basin) is another new locality for pumpkinseed in Ukraine and represents the furthest upstream population reported to date from the Dnieseter basin, the species having been previously recorded in the middle reach and the delta and estuary [75,76,77]. The population in the Heršpický pond in Czechia is also a recent invasion but of unknown origin. As noted above, the parasite community was extremely poor at both sites, which corresponds with the ‘parasite-loss theory’ of Torchin et al. [78]. While fish in the Upper Dniester were only infected by a single unidentified myxozoan found in the swim-bladder, larvae of the cestode Triaenophorus nodulosus (Pallas, 1781) were also found rarely in fish from the Heršpický pond (see Table 2). Like Burshtyn Reservoir and Heršpický Pond, the parasite community at Římov Reservoir comprised just two local species (Acanthocephalus lucii Müller, 1776, Argulus foliaceus), though at higher prevalence than the other sites. These data tend to agree with the ‘enemy release hypothesis’, which explains the success of early invasions through temporary release from parasites and pathogens [78,79,80].
Parasite acquisition by pumpkinseed in both regions studied was generally low, particularly at localities with recently established pumpkinseed populations, which agrees with findings from other European water bodies. In comparison, the older, established population in the Kolín oxbow, which was introduced ca. 15 years before this investigation, was characterised by a high parasite diversity and equitability, low dominance and highest species richness at both component and infracommunity levels. Similar examples of temporal acquisition of local parasites have been described for other non-native fish species, e.g. round goby (Neogobius melanostomus (Pallas, 1814)) [15, 81]. Our data, showing high diversity and equitability at the Kolín oxbow, therefore, indicate that 15 years after population establishment, the parasite community reached certain stability, although being composed of acquired parasites only. The component community species richness of nine spp. was only slightly lower compared to old-established populations in Germany and France, where 10–11 spp. were found using the same parasite collection methodology and sample size [42].
The species richness of co-introduced North American parasites was also generally low when compared to older European populations in France and Germany [42]. For example, five co-introduced parasite species, Actinocleidus oculatus (Mueller, 1934), Actinocleidus recurvatus Mizelle & Donahue, 1944, O. dispar, O. similis and P. centrarchi, showed high abundance in the Rhine basin in Germany [43], while the same species were observed in France accompanied by Cleidodiscus robustus Mueller, 1934, Gyrodactylus macrochiri Hoffman & Putz, 1964 and Proteocephalus ambloplitis (Leidy, 1887)[33, 36]. Pumpkinseed from these regions, which represent the first area of European pumpkinseed introduction [82], exhibit high genetic diversity alongside their high North American parasite diversity [42]. Thus, the low parasite load of co-introduced parasites in more recent populations in Czechia and the western part of Ukraine may be indicative of ‘dilution’ from the source pumpkinseed populations.
This study showed that parasite communities in recently established pumpkinseed populations in Ukraine and Czechia are less diverse than those established decades previously and that this applies to both co-introduced and acquired parasite species. The generally low parasite loads in these new populations may play an important role in their ability to successfully establish and create strong populations and may provide a competitive advantage over local species.
References
Dobson AP, Hudson PJ (1986) Parasites, disease and the structure of ecological communities. Trends Ecol Evol 1(1):11–15. https://doi.org/10.1016/0169-5347(86)90060-1
Chalkowski K, Lepczyk CA, Zohdy S (2018) Parasite ecology of invasive species: conceptual framework and new hypotheses. Trends Parasitol 34(8):655–663. https://doi.org/10.1016/j.pt.2018.05.008
Goedknegt MA, Feis ME, Wegner KM, Luttikhuizen PC, Buschbaum C, Camphuysen KCJ, van der Meer J, Thieltges DW (2016) Parasites and marine invasions: ecological and evolutionary perspectives. J Sea Res 113:11–27. https://doi.org/10.1016/j.seares.2015.12.003
Sarabeev V, Balbuena JA, Desdevises Y, Morand S (2022) Host-parasite relationships in invasive species: macroecological framework. Biol Invasions 24:2649–2664. https://doi.org/10.1007/s10530-022-02821-7
Taraschewski H (2006) Hosts and parasites as aliens. J Helminthol 80:99–128. https://doi.org/10.1079/JOH2006364
Ondračková M, Dávidová M, Přikrylová I, Pečínková M (2011) Monogenean parasites of introduced pumpkinseed Lepomis gibbosus (Centrarchidae) in the Danube River Basin. J Helminthol 85(4):435–441. https://doi.org/10.1017/S0022149X10000805
Ondračková M, Seifertová M, Bryjová A, Leis E, Jurajda P (2020) Morphometric and genetic evidence for cryptic diversity in Gyrodactylus (Monogenea) infecting non-native European populations of Ameiurus nebulosus and A. melas. Parasitology 147(14):1700–1711. https://doi.org/10.1017/S0031182020001195
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol Parasites Wildl 3(2):171–177. https://doi.org/10.1016/j.ijppaw.2014.04.002
Šimková A, Řehulková E, Rasoloariniaina JR, Jorissen MWP, Scholz T, Faltýnková A, Mašová Š, Vanhove MPM (2019) Transmission of parasites from introduced tilapias: a new threat to endemic Malagasy ichthyofauna. Biol Invasions 21:803–819. https://doi.org/10.1007/s10530-018-1859-0
Mastitsky SE, Veres JK (2010) Field evidence for a parasite spillback caused by exotic mollusk Dreissena polymorpha in an invaded lake. Parasitol Res 106:667–675. https://doi.org/10.1007/s00436-010-1730-4
Faliex E, Antunes C, Bardonnet A, Costarrosa A, Diaz E, De Miguel R, Domingos I, Fernandez-Delgado C, Herrera M, Korta M, Lagarde R, Mercader M, Monteiro R, Moura A, Portela T, Prellwitz F, Regli N, Rives J, Simon G, Hernández LZ, Amilhat E (2022) Anguillicola crassus Infection in Different Ecosystems of the Southwestern European Area. Biol Life Sci Forum 13(1):141. https://doi.org/10.3390/blsf2022013141
El-Rashidy HH, Boxshall GA (2009) Parasites gained: alien parasites switching to native hosts. J Parasitol 95(6):1326–1329. https://doi.org/10.1645/GE-2190.1
Tanaka S, Nishida T, Ohsaki N (2007) Sequential rapid adaptation of indigenous parasitoid wasps to the invasive butterfly Pieris brassicae. Evol 61(8):1791–1802. https://doi.org/10.1111/j.1558-5646.2007.00165.x
Gendron AD, Marcogliese D, Thomas M (2012) Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St Lawrence basin. Biol Invasions 14:367–384. https://doi.org/10.1007/s10530-011-0083-y
von dem Borne M (1906) Teichwirtschaft, 5th edn. Paul Parey, Berlin
Kunstler J (1908) Amiurus nebulosus et Eupomotis gibbosus. Bulletin de la Société d’Acclimatation 55:238–244
Stansch K (1914) Die exotischen Zierfische in Wort und Bild. Wenzel & Sohn, Braunschweig
Copp GH, Fox MG (2007) Growth and life history traits of introduced pumpkinseed (Lepomis gibbosus) in Europe, and the relevance to invasiveness potential. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Springer, Berlin, pp 289–306
Fox MG, Copp GH (2014) Old world vs new world—life history alterations in a successful invader introduced across Europe. Oecologia 174:435–446. https://doi.org/10.1007/s00442-013-2776-7
Afanasyev SA, Gupalo YA, Manturova OV (2017) Distribution and peculiarities of biology of the pumpkinseed Lepomis gibbosus (Perciformes: Centrarchidae) in the water bodies of Kyiv City. Hydrobiol J 53(3):14–25. https://doi.org/10.1615/HydrobJ.v53.i3.20
Tarkan AS, Karakuş U, Top-Karakuş N, Keskin E, Ünal EM, Britton JR (2021) Invasion of pumpkinseed Lepomis gibbosus is facilitated by phenotypic plasticity across its invasion gradient. Biol Invasions 23:3201–3214. https://doi.org/10.1007/s10530-021-02574-9
Commission E (2019) Commission implementing regulation (EU) 2019/1262 of 25 July 2019 amending Implementing Regulation (EU) 2016/1141 to update the list of invasive alien species of Union concern. Off J Eur Union L199:1–4
Pavlov PY, Bilko VP (1962) Soniachna ryba v prydunayskykh vodoymakh. Dopovidi AN URSR 11:1514–1516 (in Ukrainian)
Diripasko OA, Demchenko NA, Kulik PV, Zabroda TA (2008) An expansion of the pumpkinseed, Lepomis gibbosus (Centrarchidae, Perciformes), area of distribution into the east of Ukraine. Vestnik Zoologii 42:269–273
Movchan YV (2011) Ryby Ukrainy. Zoloti Vorota, Kyiv (in Ukrainian)
Schäferna K (1929) Nové americké nadělení (New American mess). Rybářský věstník 9(10):147–148 (in Czech)
Hanel L, Lusk S (2005) Ryby a mihule České Republiky (Fish and lampreys of the Czech Republic). Česky svaz ochráncu prirody, Vlasim (in Czech)
Kulakovskaya OP, Koval VP (1973) Parazitofauna ryb basseyna Dunaya (Fish parasite fauna of the Danube basin). Naukova Dumka, Kiev (in Russian)
Piasecki W, Falandysz M (1994) Preliminary survey on parasite fauna of pumpkinseed sunfish. Acta Ichthyol Piscat 24:87–100. https://doi.org/10.3750/AIP1994.24.1.07
Moravec F (2001) Checklist of metazoan parasites of fishes of the Czech Republic and the Slovak Republic (1873–2000). Akademia, Praha
Goswami U, Molnár K, Cech G, Eiras JC, Bandyopadhyay PK, Ghosh S, Czeglédi I, Székely C (2021) Evidence of the American Myxobolus dechtiari was introduced along with its host Lepomis gibbosus in Europe: molecular and histological data. Int J Parasitol Parasites Wildl 15:51–57. https://doi.org/10.1016/j.ijppaw.2021.04.005
Havlátová L, Ondračková M, Přikrylová I (2015) Monogenean parasites of Lepomis gibbosus Linnaeus introduced into the River Durance. Helminthologia 52(4):323–330. https://doi.org/10.1515/helmin-2015-0051
Rubtsova NY (2015) First record of Onchocleidus dispar, an alien monogenean from introduced pumpkinseed fish Lepomis gibbosus (Pisces, Centrarchidae) in Ukraine. Sci Parasitol 16(3):83–88
Kvach Y, Matvienko N, Bryjová A, Ondračková M (2018) Aquaculture as a possible vector in the spread of Posthodiplostomum centrarchi (Hoffman, 1958) (Digenea: Diplostomidae) in Europe. BioInvasions Rec 7(4):427–432. https://doi.org/10.3391/bir.2018.7.4.12
Kvach Y, Seifertová M, Carassou L, Ondračková M (2020) First record of the American cestode Proteocephalus ambloplitis (Leidy, 1887) (Proteocephalidae) in Europe. J Helminthol 94:e144. https://doi.org/10.1017/S0022149X20000267
Kvach Y, Jurajda P, Bryjová A, Trichkova T, Ribeiro F, Přikrylová I, Ondračková M (2017) European distribution for metacercariae of the North American digenean Posthodiplostomum cf. minimum centrarchi (Strigeiformes: Diplostomidae). Parasitol Int 66:635–642. https://doi.org/10.1016/j.parint.2017.06.003
Stoyanov B, Georgieva S, Pankov P, Kudlai O, Kostadinova A, Georgiev BB (2017) Morphology and molecules reveal the alien Posthodiplostomum centrarchi Hoffman, 1958 as the third species of Posthodiplostomum Dubois, 1936 (Digenea: Diplostomidae) in Europe. Syst Parasitol 94:1–20. https://doi.org/10.1007/s11230-016-9680-6
Stoyanov B, Mutafchiev Y, Pankov P, Georgiev BB (2017) Helminth parasites in the alien Lepomis gibbosus (L.) (Centrarchidae) from the Lake Atanasovsko wetlands, Bulgaria: survey of species and structure of helminth communities. Acta Zool Bulg 69(4):555–574
Hockley FA, Williams CF, Reading AJ, Taylor NGH, Cable J (2011) Parasite fauna of introduced pumpkinseed fish Lepomis gibbosus: first British record of Onchocleidus dispar (Monogenea). Dis Aquat Org 97:65–73. https://doi.org/10.3354/dao02402
Masson G, Vanacker M, Fox M, Beisel J (2015) Impact of the cestode Triaenophorus nodulosus on the exotic Lepomis gibbosus and the autochthonous Perca fluviatilis. Parasitology 142:745–755. https://doi.org/10.1017/S0031182014001826
Ondračková M, Bartáková V, Kvach Y, Bryjová A, Trichkova T, Ribeiro F, Carassou L, Martens A, Masson G, Zechmeister T, Jurajda P (2021) Parasite infection reflects host genetic diversity in the pumpkinseeds non-native range. Hydrobiologia 848:2169–2187. https://doi.org/10.1007/s10750-020-04410-y
Ondračková M, Kvach Y, Martens A, Jurajda P (2019) Limited parasite acquisition by non-native Lepomis gibbosus (Antinopterygii: Centrarchidae) in two ponds at the Upper Rhine in Germany. J Helminthol 93(4):453–460. https://doi.org/10.1017/S0022149X18000469
Moshu A (2012) Protistan parasites (Protista) of the pumpkinseed, Lepomis gibbosus (L., 1758) (Perciformes, Centrarchidae), from Prut-Dniester hydrographic interfluvial space. In: Materials of the international conference “modern problems of general parasitology” (Moscow, October 30-November 1, 2012). Moscow, pp 225–229 (in Russian with English summary)
Gologan I (2020) The helminth fauna of some invasive fishes from various natural and artificial water bodies from the Republic of Moldova. Lucrări Ştiinţifice Seria Medicină Veterinară 63(2):136–141
Ondračková M, Tkachenko M, Bartáková V, Bryjová A, Janáč M, Zięba G, Pyrzanowski K, Kvach Y (2023) Population genetic structure, parasite infection and somatic condition of pumpkinseed Lepomis gibbosus (Actinopterygii: Centrarchidae) in the Oder river basin. J Fish Biol 102(2):426–442. https://doi.org/10.1111/jfb.15273
Djikanović V, Simonović P, Cakić P, Nikolić V (2018) Parasitofauna of allochthonous fish species in the open waters of the Danube River basin (Serbian part)—impact on the native fish fauna. Ecol Environ Res 16:6129–6142. https://doi.org/10.15666/aeer/1605_61296142
Kvach Y, Tkachenko M, Bartáková V, Kutsokon Y, Janáč M, Demchenko V, Ondračková M (2023) Parasite communities and genetic structure of non-native pumpkinseed, Lepomis gibbosus, in different Black Sea drainages of Ukraine. Knowl Manag Aquat Ecosyst 424:1. https://doi.org/10.1051/kmae/2022023
Ondračková M, Fojtů J, Seifertová M, Kvach Y, Jurajda P (2019) Non-native parasitic copepod Neoergasilus japonicus (Harada, 1930) utilizes non-native fish host Lepomis gibbosus (L.) in the floodplain of the River Dyje (Danube basin). Parasitol Res 118(1):57–62. https://doi.org/10.1007/s00436-018-6114-1
Yuryshynets V, Ondračková M, Kvach Y, Masson G (2019) Trichodinid ectoparasites (Ciliophora: Peritrichia) of non-native pumpkinseed (Lepomis gibbosus) in Europe. Acta Protozool 58(2):69–79. https://doi.org/10.4467/16890027AP.19.009.11418
Kvach Y, Tkachenko M, Seifertová M, Ondračková M (2021) Insights into the diversity, distribution and phylogeny of three ergasilid copepods (Hexanauplia: Ergasilidae) in lentic water bodies of the Morava River basin, Czech Republic. Limnologica 91:125922. https://doi.org/10.1016/j.limno.2021.125922
Kovalchuk I (2004) Burshtynske Vodoskhovyshche. In: Dzhiuba IM, Zhukovskyi AI, Zhelezniak MH (eds) Encyclopedia Suchasnoi Ukrainy of Modern Ukraine. NASU Institute of Encyclopaedic Research, Kyiv. https://esu.com.ua/article-38195
Greben VV, Khilchevskii VK, Stashuk VA, Chunaryov OV, Yaroshevych OY (2014) Vodnyi fond Ukrainy: Shtuchni vodoymy—vodoskhovyshcha i stavky (The water fund of Ukraine: artificial reservoirs—reservoirs and ponds). Interpress, Kyiv (in Ukrainian)
Říha M, Kubečka J, Vašek M, Seďa J, Mrkvička T, Prchalová M, Matěna J, Hladík M, Čech M, Draštík V, Frouzová J, Hohausová E, Jarolím O, Jůza T, Kratochvíl M, Peterka J, Tušer M (2009) Long-term development of fish populations in the Římov Reservoir. Fish Manag Ecol 16:121–129. https://doi.org/10.1111/j.1365-2400.2008.00650.x
Kvach Y, Ondračková M, Janáč M, Jurajda P (2016) Methodological issues affecting the study of fish parasites. I. Duration of live fish storage prior to dissection. Dis Aquat Org 119:107–115. https://doi.org/10.3354/dao02990
Malmberg G (1970) The excretory systems and the marginal hooks as a basis for the systematics of Gyrodactylus (Trematoda, Monogenea). Arkiv för Zoologi 23:1–235
Georgiev B, Biserkov V, Genov T (1986) In toto staining method for cestodes with iron acetocarmine. Helminthologia 23:279–281
Cribb TH, Bray RA (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst Parasitol 76:1–7. https://doi.org/10.1007/s11230-010-9229-z
Bush AO, Lafferty KD, Lotz JM, Shostak AWParasitology meets ecology on its own terms: Margolis, et al (1997) revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Sørensen TA (1948) A new method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analysis of vegetation on Danish commons. Kongelige Danske Videnskabernes Selskabs (Biologiske Skrifter) 5:1–34
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):4
Luton K, Walker D, Blair D (1992) Comparison of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol Biochem Parasitol 56:323–328. https://doi.org/10.1016/0166-6851(92)90181-I
Bowles J, Hope M, Tiu WU, Liu XS, McManus DP (1993) Nuclear and mitochondrial genetic markers highly conserved between Chinese and Philippine Schistosoma japonicum. Acta Trop 55:217–229. https://doi.org/10.1016/0001-706X(93)90079-Q
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Locke SA, McLaughlin JD, Marcogliese DJ (2010) DNA barcodes show cryptic diversity and a potential physiological basis for host specificity among Diplostomoidea (Platyhelminthes: Digenea) parasitizing freshwater fishes in the St. Lawrence River. Canada Mol Ecol 19:2813–2827. https://doi.org/10.1111/j.1365-294X.2010.04713.x
Cech G, Sándor D, Molnár K, Paulus P, Papp M, Preiszner B, Vitál Z, Varga A, Cs S (2020) New record of metacercariae of the North American Posthodiplostomum centrarchi (Digenea, Diplostomidae) in pumpkinseed (Lepomis gibbosus) in Hungary. Acta Vet Hung 68(1):20–29. https://doi.org/10.1556/004.2020.00001
Miller JH (1954) Studies on the life history of Posthodiplostomum minimum (MacCallum 1921). J Parasitol 40:255–270. https://doi.org/10.2307/3273737
Sterud E, Jørgensen A (2006) Pumpkinseed Lepomis gibbosus (Linnaeus, 1758) (Centrarchidae) and associated parasites introduced to Norway. Aquat Invasions 1(4):278–280. https://doi.org/10.3391/ai.2006.1.4.10
Galli P, Stefani F, Benzoni F, Crosa G, Zullini A (2003) New records of alien monogeneans from Lepomis gibbosus and Silurus glanis in Italy. Parassitologia 45(3–4):147–149
Kvach Y, Ondračková M, Kutsokon Y, Dzyziuk N (2018) New record of monogenean parasites on non-indigenous fishes in the Ukrainian Danube delta. BioInvasions Rec 7:65–72. https://doi.org/10.3391/bir.2018.7.1.10
Kurtyak F, Ye T, Stegun V, Velikopolskiy I (2009) Review of finfishes of river of Latorica basin within the limits of Ukraine. Visnyk of Lviv Univ (Biol Ser) 50:85–94 (in Ukrainian with English summary)
Didenko A, Velykopolsky I, Chuklin A (2014) Use of Poacher’s catches for studying fish fauna in the water bodies of the Transcarpathian region (Ukraine). Transylv Rev Syst Ecol Res 16(2):107–116. https://doi.org/10.1515/trser-2015-0019
Zięba G, Dukowska M, Przybylski M, Fox MG, Smith C (2018) Parental care compromises feeding in the pumpkinseed (Lepomis gibbosus). Sci Nat 105:26. https://doi.org/10.1007/s00114-018-1554-0
Zięba G, Vilizzi L, Copp GH (2020) How likely is Lepomis gibbosus to become invasive in Poland under conditions of climate warming? Acta Ichthyol Piscat 50(1):37–51. https://doi.org/10.3750/AIEP/02390
Bulat D, Bulat D, Toderaş I, Usatîi M, Fulga N, Dumbrăveanu D, Rusu V (2013) Particularităţile ecologice ale speciei invazive Lepomis gibbosus (Linnaeus, 1758) în limitele Republicii Moldova (The ecological peculiarities of the invasive species Lepomis gibbosus (Linnaeus, 1758) within the limits of the Republic of Moldova). Mediul Ambiant 4(70):19–26 (in Romanian)
Snigirov S, Kvach Y, Goncharov O, Sizo R, Sylantyev S (2019) Hydrology and parasites: what divides the fish community of the lower Dniester and Dniester estuary into three? Estuar Coast Shelf Sci 217:120–131. https://doi.org/10.1016/j.ecss.2018.11.022
Mustea M, Filipenco S, Bulat D (2023) Particularitățile biologice ale bibanului-soare—Lepomis gibbosus (Linnaeus, 1758) din lacul refrigerent Cuciurgan (The biological peculiarities of the sun perch—Lepomis gibbosus (Linnaeus, 1758) from the Cuciurgan cooling lake). Studia Universitatis Moldaviae (Seria Ştiinţe Reale şi ale Naturii) 1(171):83–90. https://doi.org/10.59295/sum1(171)2023_11. (in Romanian)
Torchin ME, Lafferty K, Dobson A, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–629. https://doi.org/10.1038/nature01346
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Heger T, Jeschke JM (2014) The enemy release hypothesis as a hierarchy of hypotheses. Oikos 123:741–750. https://doi.org/10.1111/j.1600-0706.2013.01263.x
Ondračková M, Valová Z, Hudcová I, Michálková V, Šimková A, Borcherding J, Jurajda P (2015) Temporal effects on host-parasite associations in four naturalized goby species living in sympatry. Hydrobiologia 746:233–243. https://doi.org/10.1007/s10750-014-1967-5
Wiesner C, Wolter C, Rabitsch W, Nehring S (2010) Gebietsfremde Fische in Deutschland und Österreich und mögliche Auswirkungen des Klimawandels. Bundesamt für Natuschutz, Bonn
Acknowledgements
The authors thank their colleagues from the Czech Academy of Sciences (Institute of Vertebrate Biology) for support during fish sampling in Czechia and parasite processing, along with Myroslav Markovych (Uzhhorod National University, Uzhhorod, Ukraine) for his help in the sampling of fish in the village of Myhai. We also thank Veronika Bartáková for her help with sequence processing and Michal Hnilička for her help with creating the maps. Finally, we would like to thank Kevin Roche for proofreading the English text.
Funding
Open access publishing supported by the National Technical Library in Prague. This study was financially supported through Inter-Action Project LTAUSA19092, funded by the Ministry of Education, Youth and Sports of the Czech Republic, and by Czech Science Foundation Project No. 20-29111S.
Author information
Authors and Affiliations
Contributions
The first manuscript draft was prepared by ID, who also sampled and dissected all fish from Ukraine, identified most of them to species level, prepared the datasets and provided the descriptive statistical analysis. Sampling in Ukraine was organised by KN and ID. Conceptualisation of the Ukrainian part of the study was performed by YK and KN, while the initial idea was provided by MO. Sampling, dissection and parasite identification in Czechia were provided by MO, MT and YK. The DNA analysis of digeneans was provided by MT. Funding acquisition was performed by MO, who also provided the final statistical analysis. The review and editing process was performed by all authors and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All national and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dudliv, I., Kvach, Y., Tkachenko, M.Y. et al. Comparative Analysis of Parasite Load on Recently Established Invasive Pumpkinseed Lepomis gibbosus (Actinopterygii: Centrarchidae) in Europe. Acta Parasit. 69, 819–830 (2024). https://doi.org/10.1007/s11686-024-00794-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-024-00794-2