Abstract
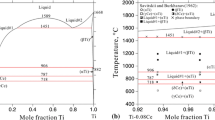
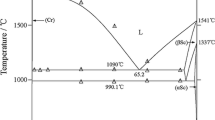
The Ge-Ti binary phase diagram was remodeled using the CALPHAD approach implementing newly available experimental data and data from ab-initio calculations. The modelled phase diagram is based on a recently published experimental phase diagram, enthalpy of formation calculated by ab-initio methods, experimentally measured heat capacity of phases Ge3Ti5 and Ge5Ti6, enthalpy of formation of Ge3Ti5 at 298 K and enthalpy of mixing of liquid at 2000 K. A very good agreement with the experimental results was reached for Ge-Ti phase diagram and for the calculated thermodynamic properties, namely the enthalpy of mixing of the liquid phase at 2000 K and heat capacity of phases Ge3Ti5 and Ge5Ti6.








Similar content being viewed by others
References
D. Liu, H. Yan, X. Yuan, Y. Chung, Y. Du, H. Xu, L. Liu, and P. Nash, Thermodynamic Modeling of the Ge-Ti System Supported by Key Experiment, Thermochim. Acta, 2011, 521, p 148–154.
R.W. Bittner, C. Collinet, J.C. Tedenac, and K.W. Richter, Revision of the Ge-Ti Phase Diagram and Structural Stability of the New Phase Ge4Ti5, J. Alloys Compd., 2013, 577, p 211–216.
M.V. Rudometkina, Y.D. Seropegin, A.V. Gribanov, and L.S. Gusei, Phase Equilibria in the Titanium-Niobium-Germanium System at 1170 K, J. Less-Common Met., 1989, 147, p 239–247.
T.B. Massalski, H. Okamoto, P.R. Subramanian, L. Kacprczak, Binary Alloy Phase Diagrams, 2nd Ed., ASM, Materials Park, Ohio, (1990)
J. Wirringa and M. Binnewies, Chemical Vapor Transport of Intermetallic Systems, Part 8. Chemical Transport of Titanium Germanides, Z. Anorg. Allg. Chem., 2000, 626, p 996–998.
A.N. Shlapak, E.A. Beloborodova, and G.I. Batalin, Enthalpies of Mixing of Binary Molten Alloys of Germanium with Vanadium and Titanium, Zh. Fiz. Khim., 1978, 52(8), p 2097–2099.
Y.O. Esin, M.G. Valishev, A.F. Ermakov, P.V. Geld, and M.S. Petrushevskii, Enthalpies of Formation of Molten Germanium and Nickel Alloys With Titanium, Zhurnal Fizicheskoi Khimii, 1981, 55(3), p 753–754.
A. Yassin, M. Gilbert, and R. Castanet, Enthalpies of Formation of Binary Systems of Ti, V, Mo and Hf with Ge, J. Alloys Compd., 2001, 322, p L19–L22.
O.J. Kleppa and W.G. Jung, Standard Enthalpies of Formation of Metal Germanides (M5Ge3; M=Titanium, Vanadium, Manganese, Iron, Cobalt, Nickel) by High Temperature Calorimetry, High Temp. Sci., 1990, 29(2), p 109–123.
E.V. Belokurov, G.I. Kalishevich, and P.V. Geld, Heat Capacity, Standard Enthalpies and Entropies of Titanium Germanide (Ti5Ge3) and Scandium Germanide (Sc5Ge3), Zh. Fiz. Khim., 1978, 52(11), p 2970–2971.
S. Zarembo, R.J. Kematick, C.E. Myers, and E.J. Cotts, Vaporization Thermodynamics and Heat Capacities of Ti5Ge3 and Ti6Ge5, J. Alloys Compd., 2000, 306, p 78–86.
N. Saunders and A.P. Miodownik, Calphad (A Comprehensive Guide). Pergamon Press, Oxford, 1998.
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics: The Calphad Method. Cambridge University Press, New York, 2004.
W. Cao, S.L. Chen, F. Zhang, K. Wu, Y. Yang, Y.A. Chang, R. Schmid-Fetzer, and W.A. Oates, PANDAT Software with PanEngine PanOptimizer and PanPrecipitation for Multicomponent Phase Diagram Calculation and Materials Property Simulation, Calphad, 2009, 33, p 328.
J.-O. Andersson, T. Helander, L. Hoglund, P. Shi, and B. Sundman, Thermo-calc and DICTRA, Computational Tools for Materials Science, Calphad, 2002, 26, p 273–312.
SGTE Unary Database, Version 4.4, www.sgte.org. Accessed (2020)
M.I. McQuillan, A Study of the Titanium–Germanium System in the Region 0-11 Atomic Percent Germanium, J. Inst. Metals, 1955, 83, p 485–489.
O.K. Belousov and I.I. Kornilov, The Solubility of Germanium in Titanium, Izv. Akad. Nauk SSSR Met., 1976, 1, p 168–169.
V.C. Petersen and R.W. Huber, The Titanium–Germanium System from 0 to 30% Germanium, U.S. Bur. Mines Rept. Invest., 1957, 5365, p 20.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zobač, O., Kroupa, A. CALPHAD-Based Thermodynamic Description of the Binary Phase Diagram Ge-Ti. J. Phase Equilib. Diffus. 44, 115–126 (2023). https://doi.org/10.1007/s11669-023-01028-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01028-0