Abstract
Aims
Studies on tree CH4 exchange in boreal forests regarding seasonality and role of tree canopies are rare. We aimed to quantify the contribution of boreal trees to the forest CH4 budget during spring leaf-out and to reveal the role of microbes in the CH4 exchange.
Methods
Methane fluxes of downy birch and Norway spruce (Betula pubescens and Picea abies) growing on fen and upland sites were measured together with soil CH4 flux, environmental variables and microbial abundances involved in the CH4 cycle. Tree CH4 fluxes were studied from three stem heights and from shoots.
Results
The trees emitted CH4 with higher stem emissions detected from birch and higher shoot emissions from spruce. The stem CH4 emissions from birches at the fen were high (mean 45 µg m−2 h−1), decreasing with stem height. Their dynamics followed soil temperature, suggesting the emitted CH4 originated from methanogenic activity, manifested in high mcrA gene copy numbers, in the peat soil. Methanogens were below the quantification limit in the tree tissues. Upscaled tree CH4 emissions accounted for 22% of the total CH4 emissions at the fen.
Conclusions
The variation in stem CH4 flux between the trees and habitats is high, and the emissions from high-emitting birches increase as the spring proceeds. The lack of detection of methanogens or methanotrophs in the aboveground plant tissues suggests that these microbes did not have a significant role in the observed tree-derived fluxes. The stem-emitted CH4 from birches at the fen is presumably produced microbially in the soil.
Similar content being viewed by others
Introduction
Methane (CH4) is one of the most abundant greenhouse gases with atmospheric mixing ratio of 1.803 ppm (Hartmann et al. 2013). The quantity of atmospheric CH4 has increased over 1.5-fold since preindustrial times and continues to rapidly increase (Hartmann et al. 2013). Anthropogenic sources of CH4 are considered to be well-known, while cycling of CH4 in terrestrial ecosystems and its natural sources remain poorly quantified (Conrad 2009; Xu et al. 2016). During the last few decades the importance of plants in CH4 cycling in different ecosystems has been recognized, and much attention has been directed to revealing their role in global CH4 dynamics (Carmichael et al. 2014). Numerous studies have shown that trees are capable of emitting CH4 from their stems (Terazawa et al. 2007; Pangala et al. 2013, 2014, 2015, 2017; Machacova et al. 2016; Wang et al. 2016).
Most of the research on tree CH4 exchange has been performed on species from tropical or temperate vegetation zones (e.g. Gauci et al. 2010; Pangala et al. 2015, 2017; Wang et al. 2016; Pitz & Megonigal 2017; Plain et al. 2019), while studies in boreal forests are still rare (Machacova et al. 2016). In general, trees growing in wetland forests have acquired more attention as they are known to be hotspots of CH4 production where trees act as conduits for soil-produced CH4 (Pangala et al. 2013, 2014, 2017; Jeffrey et al. 2020a; Schindler et al. 2020; Sjögersten et al. 2020; Mander et al. 2021; Moldaschl et al. 2021). Leaf-level CH4 fluxes of tree canopies are much less studied, and the few studies worldwide indicate variation between small emission (Machacova et al. 2016; Pangala et al. 2017) and small uptake (Sundqvist et al. 2012; Putkinen et al. 2021), or zero fluxes (Takahashi et al. 2012), while the processes remain unknown.
The observed connections between the stem CH4 fluxes and soil wetness (Rusch and Rennenberg 1998; Machacova et al. 2013, 2016; Maier et al. 2018; Barba et al. 2019a) have led researchers to set a primary hypothesis that the CH4 emitted from trees is produced by methanogens in anaerobic soils and microsites, taken up by tree roots, transported into the aboveground tree tissues and released into the atmosphere (Rusch and Rennenberg 1998). In this way trees may act as pathways for the soil-produced CH4 instead of the CH4 being oxidized by methanotrophic microbes on its way through the aerated soil layers (Bender and Conrad 1993; Conrad 2009). Moreover, CH4 emitted from trees might be produced within the tree tissues itself, by microorganisms living in plant tissues (Zeikus and Ward 1974; Covey et al. 2012; Yip et al. 2019; Putkinen et al. 2021) and/or by plant physiological processes (Keppler et al. 2006). On the other hand, initial studies indicate also a possible CH4 oxidation potential in tree tissues (Jeffrey et al. 2021a, b).
Aerobic CH4 production in plants has mostly been studied in laboratory conditions with herbaceous plants. Aerobic CH4 emissions from herbaceous as well as woody plants have been suggested to occur especially during the growth and decay of new plant cells, linking the CH4 formation to its identified CH4 precursors such as lignin, cellulose, pectin and methionine (Keppler et al. 2008; Vigano et al. 2008; Messenger et al. 2009; Althoff et al. 2014; Fraser et al. 2015; Lenhart et al. 2015; Benzing et al. 2017). The importance of the processes linked to aerobic CH4 production and emissions in nature are not yet fully understood.
Boreal upland forests are commonly considered a net sink for atmospheric CH4 due to microbial CH4 oxidation in soil, whereas the role of trees in the CH4 dynamics has received relatively little attention. Boreal trees have been observed to both emit (Machacova et al. 2016; Tenhovirta et al. 2022) and uptake (Sundqvist et al. 2012; Putkinen et al. 2021) atmospheric CH4, and these contradictory results call for more research. Furthermore, very little is known of the seasonality and the effects of tree physiological activity and environmental drivers on CH4 flux dynamics of tree stems and canopies.
In this study, we measured the CH4 fluxes of the stems and shoots of two common boreal tree species, downy birch (Betula pubescens Ehrh.) and Norway spruce (Picea abies (L.) H. Karst.), as well as of the forest floor, during the spring leaf-out period. Downy birch has distribution from Scandinavia to Central Europe and to Eastern Siberia, while Norway spruce grows throughout Scandinavia and eastern parts of Europe limiting to Ural Mountains. We aim to I) evaluate the effect of the leaf-out period to the CH4 exchange of the tree leaves and stems, II) assess the contribution of these tree species to the CH4 cycle within a boreal forest ecosystem, and III) to connect the CH4 fluxes to meteorological data, soil and tree-physiological parameters, and the abundance of the CH4 producing and oxidizing microbes in the soil, within the trees and in the ground vegetation.
Materials and methods
Site description and experimental design
The tree CH4 exchange measurements were conducted at the SMEAR II station (Station for Measuring Forest Ecosystem-Atmosphere Relations) in Hyytiälä, southern Finland (61°51´N, 24°17´E, 181 m a.s.l.) (Hari and Kulmala 2005). Mean annual temperature and precipitation of the area are 3.5 °C and 711 mm, respectively (in 1981–2010) (Pirinen et al. 2012).
Two experimental plots differing in their forest structure and soil type and characteristics were selected within the studied forest: one situated on organic and the other on mineral soil. The organic soil site is a small (ca. 300 m2) mesotrophic forested fen with approximately 0.6 m thick peat layer and had a water table level (WTL) located 5–10 cm below the surface during the study period. Trees are unevenly aged and the main tree species in the highest canopy level is Norway spruce with downy birch as mixed species. The hollows are dominated by Sphagnum girgensohnii while hummocks are covered with mix of Pleurozium schreberi, Polytrichum commune and Hylocomium splendens (Table 1). The upland mineral soil site is located in a Scots pine (Pinus sylvestris) dominated stand established in 1962 (Hari and Kulmala 2005). The soil water content at the upland site was on average 0.28 m3 m−3 during the study period. Downy birch and Norway spruce occur as mixed species and with Scots pine they form an evenly aged canopy layer. Understory is a sparse mix of downy birch, Norway spruce and rowan (Sorbus aucuparia). (The main species in the field and ground layers are listed in Table 1.)
We measured stem and shoot CH4 fluxes from five trees in total: at the fen site a scaffold tower was placed so that we had access to two mature downy birches (trees number 1 and 2) and two Norway spruces (trees no. 3 and 4), and at the upland site we had access to one downy birch (tree no. 5). The shoot measurements required access to the canopy, limiting the selection of sample trees. Spruce shoots were not within range of the scaffold tower at the upland site. At the forested fen site the height of the studied trees varied between 8.5–12.9 m and the stem diameter between 0.073–0.125 m (diameter at breast height, DBH). The birch at the upland site was 19.6 m of height and 0.195 m of diameter (Table 2). Based on a forest inventory we made, the number of birch and spruce stems at the fen site were 2267 ha−1 and 1400 ha−1, respectively, and the number of birches at the upland site was estimated to be 200 ha−1 (including all the trees higher than 1.3 m; Table 2).
The fluxes were measured from three stem heights, and from 1–3 shoots per tree (Table 3). The forest floor CH4 fluxes were measured from three points at the fen site and from one point at the upland site. The CH4 flux measurement campaign was conducted in the beginning of the growing season from 28 April to 11 June 2015. During the campaign, the CH4 exchange measurements of the sample trees and the forest floor were conducted simultaneously twice a week at the fen and weekly at the upland forest site, except for the upland forest floor, which had semi-automated daily measurements. The measurements were mainly conducted during daytime (between 9 a.m. and 3 p.m.), complemented with five night-time measurements (between 9 p.m. and 5 a.m.) in May (on 5th, 6th, 11th, 20th, and 25th of May). All the measurements on a site were conducted within one day, and the sites were measured on successive days.
Chamber design
The closed static chamber method (non-steady-state chambers) as described by Livingston & Hutchinson (1995) was used to determine the CH4 exchange of the trees and the forest floor. Different types of stem chambers were used at the two sites. The stem chambers used at the forested fen site covered ca. 0.3 m of the stems cylindrically, enclosing a stem surface area of 0.055–0.14 m2 (system volume 2.9–5.2 L; both volume and stem surface area depends on trees´ DBH; modified from Machacova et al. 2016). At the upland site each stem chamber system consisted of two plastic boxes with airtight lids (Lock & Lock, Anaheim, CA, USA) interconnected with tubing (system volume 1.5 L, enclosed stem surface area in system 0.0094–0.0099 m2; Machacova et al. 2017, 2019). The shoot chambers were cylindrical in shape with FEP (fluorinated ethylene propylene) foil walls and enclosed ca. 0.3 m of the branch from the tip of the shoot (system volume 5.2 L; Machacova et al. 2016). The total leaf area in the shoot chambers at the end of the measurement campaign ranged between 0.038–0.15 m2 for the birches and 0.0064–0.021 m2 for the spruces. As the campaign was launched in the beginning of the growing season, we estimated the change of the leaf area and considered it in flux calculations. The air circulation in both stem and shoot chamber systems was ensured by fans and/or pumps (V1500-GAS-12 V standard vacuum pumps, flow rate 1.1 l/min, Xavitech, Sweden; NMP 850.1.2. KNDC B, flow rate 8.1 l/min, KNF Neuberger, Germany).
The forest floor CH4 fluxes were measured with chambers made of aluminium or stainless steel, consisting of a permanent collar installed in the soil and an upper chamber that was closed on top of the collar. At the forested fen site there were three manual soil chambers near the sample trees (total volume 102 L, enclosed soil surface area 0.30 m2; Vainio et al. 2021). The species composition inside the chambers at the fen site consisted of Sphagnum girgensohnii, P. commune, Carex digitata, Vaccinium myrtillus, Trientalis europaea, Equisetum sylvaticum, and Potentilla palustris. At the upland site, one semi-automatic soil chamber measured the CH4 flux once per day during 1–31 May (total volume 83 L, enclosed soil surface area 0.32 m2). The plant species composition in the chamber consisted of V. myrtillus, Vaccinium vitis-idaea, P. commune, Deschampsia flexuosa, P. schreberi, and H. splendens. The soil chambers were equipped with a fan to ensure mixing of the headspace air, and a vent-tube to minimize pressure disturbances.
Methane flux measurements
When measuring the stem and shoot chambers, nine gas samples were taken at time intervals of ca. 1, 30, 60, 90, 120, 160, 200, 250 and 300 min after closing the chamber. Gas samples of 20 ml were taken manually with syringes (BD Plastik™, Becton, Dickinson and Company, New Jersey, USA) and transferred into evacuated vials (12 ml, Labco Extentainer®, Labco Limited, Wales, UK). During direct sunlight, the shoot chambers were shaded with white sheets to avoid overheating of the chamber air. The headspace temperature in the shoot chambers was recorded continuously for the flux-calculation purposes with temperature sensors connected to a logger (DL2 e data logger, Delta-T Devices, Cambridge, England).
Sampling times of the soil chambers at the forested fen site were 1, 5, 15, 25, 55 and 75 min after the chamber closure, when samples of 65 ml were taken (with syringes) and 20 ml was inserted into non-evacuated vials after flushing them with the sample gas. At the upland site, the semi-automatic chamber closed automatically and injected the gas samples into evacuated vials at 1, 5, 10, 20, 30 and 50 min after closing the chamber. Chamber headspace temperature was recorded at each sampling of the soil chambers (DT-612, CEM Instruments, Shenzhen Everbest Machinery Industry Co. Ltd., Shenzhen, China). All the gas samples were stored at + 5 °C in dark before analysis.
Analysis of the CH4 concentration and the flux calculations
The CH4 and CO2 concentrations of the samples were analysed with a gas chromatograph (GC) (7890A, Agilent Technologies, California, USA) with a flame ionization detector (FID) and methanizer for CO2 (Pihlatie et al. 2013). The method quantification limit (MQL) (Corley 2003) of the GC was estimated to be 0.10 ppm as a change in the CH4 concentration, and 151 ppm for CO2.
In order to detect and omit the outliers from the CH4 concentration data, we performed a robust linear regression analysis that uses iteratively reweighted least squares with a bisquare weighting function (Holland & Welsch 1977; MATLAB R2014a). The concentration points that were given a weight value below 0.9 by the robust linear model were regarded as outliers and removed from the concentration data (Vainio et al. 2021). The CH4 fluxes were then calculated from the outlier-filtered data with linear fit (for the calculation, see Pihlatie et al. 2013) in relation to the tree stem surface area, the forest floor area, and for the shoots per dry weight (DW) as well as per leaf area. The fluxes were further flagged based on the MQL, NRMSE (Normalized Root Mean Square Error), and R2 (coefficient of determination): 1) stem and shoot fluxes above the MQL were accepted in the final data when NRMSE ≤ 0.3 and R2 ≥ 0.5, otherwise they were omitted; 2) forest floor fluxes above the MQL were accepted in the final data when NRMSE ≤ 0.2 and R2 ≥ 0.7, otherwise they were omitted; 3) fluxes below the MQL were accepted as such, as neither of the NRMSE or R2 work for close-to-zero fluxes and omitting them would distort the data (see also Vainio et al. 2021). The forest floor CH4 fluxes were further filtered based on the CO2 flux data: the closures in which the CO2 flux remained below the MQL, had NRMSE value higher than 0.1, or in which the CO2 concentration decreased during the measurement were removed from the data. In all the stem measurements, the CO2 concentration increased above the MQL. As a result, the final data comprised 88%, 93%, and 88% of the measured stem, shoot, and forest floor fluxes, respectively. In the final data, 56% of the stem fluxes, 80% of the shoot fluxes, and 42% of the forest floor fluxes were below MQL (see also Table 3).
Upscaling
To be able to compare the flux rates between the tree stems, shoots, and the forest floor, and in order to assess the total CH4 budget of the forested fen site, the measured CH4 fluxes from the trees and the forest floor were upscaled to the ecosystem level (mg CH4 ha−1 h−1). Upland forest was ruled out of the upscaling comparison since on the upland site we could measure only one sample tree. We first calculated the average stem surface area and crown biomass for both tree species separately, based on the height and the stem diameter of the trees at the fen site. For the average stem surface area, the tree was assumed as a cone, while the average crown biomass was calculated using equations by Repola (2008, 2009). For the spruces, the mean CH4 flux of all the stem measurement heights was then upscaled for a single average-tree stem area, which was then multiplied by the number of trees per species per hectare (based on the inventory at the sites). The stem fluxes of the birches showed an exponential trend regarding the tree height (CH4 emissions decreasing with height), and thus we fitted an exponential function to the flux data. We then calculated an average flux for the birches by integrating the function from the ground level to the highest measured level, upscaled the average flux for the average-tree stem area and multiplied by the number of birches at the site.
Environmental variables
Air temperature at 4.2 m above the ground (Pt 100), precipitation (18 m above ground, FDP12P, Vaisala, Finland), UV radiation (501A, Solar Light, USA), photosynthetically active radiation (PAR; Li-190SZ, Li-Cor, USA), soil water content in A horizon (0.02–0.06 m depth in the mineral soil, mean of five locations; TDR-100, Campbell Scientific Inc., USA), and soil temperature in A horizon (0.02–0.05 m depth in the mineral soil, mean of five locations; KTY81-110, Philips, NL) are continuously measured at the SMEAR II (available in SmartSMEAR database; Junninen et al. 2009), and these data were used as ancillary data for the upland site. For the forested fen site, soil temperature at two depths (ca. 0.05–0.10 m and 0.15–0.20 m; Thermochron iButtons, Maxim Integrated Products, USA), PAR (7–9 m above ground, Quantum sensor, Li-Cor Biosciences, USA) and sap flow of the studied trees (Granier 1987) were measured continuously during the measurement campaign. Sap flow represents an important variable describing the transpiration rate of the tree (Granier 1987; Hölttä and Kolari 2009), and was followed to investigate the relationship between the CH4 fluxes and the transpiration. Soil water content was not measured at the fen site due to waterlogged conditions.
Analyses of methanogenic and methanotrophic microbes
To evaluate the role of microbes in the CH4 flux dynamics, soil, deadwood and plant samples were collected for microbial analyses from the fen and upland sites in June 2014, and at the fen site again in June 2015. The plant samples included the most abundant ground and field layer species and trees at the sites. Tree samples included separate root, stem (bore samples including layers from sap- to heartwood) and shoot tissue samples. The field layer plants were divided into below- and aboveground parts, the upland soil into litter and humus sections, and the peat into upper (5–10 cm) and lower (30–40 cm) sections. Final samples for each soil or plant component were pooled from five subsamples. Samples were stored at − 80 °C, freeze-dried and homogenized by grinding. The DNA extraction was performed from 60 mg DW (trees) or 40 mg DW (other samples) of the sample material following the procedure used for root samples by Timonen et al. (2017). Sample type origin (fen vs. upland site), and their replicate numbers (n) are listed in Tables 4 and S1.
The abundances of methanogenic archaea and methanotrophic bacteria were measured through a quantitative PCR (qPCR) analysis based on the functional genes mcrA (coding for the α-subunit of the methyl-coenzyme M reductase) and pmoA (coding for the α-subunit of particulate methane monooxygenase), respectively, as described in Halmeenmäki et al. (2017). The primers we used were mlas/mcrA-rev (Steinberg and Regan 2008) and A189f/A650r (Holmes et al. 1995; Bourne et al. 2001) for the mcrA and pmoA genes, respectively. Amplification efficiencies of the individual qPCR runs were between 91.4% and 96.8% for the mcrA assay, and between 86.6% and 97.7% for the pmoA assay.
The smallest reliably quantified standard was 101 gene copies reaction−1 (both mcrA and pmoA assays) which was thus considered the limit of quantification. It should be noted that our pmoA-targeting approach did not cover methanotrophs lacking the pmoA gene, i.e. genus Methylocella and strains Methyloferula stellata AR4 and Methyloceanibacter methanicus R-67174 (Farhan Ul Haque et al. 2020).
Statistical analyses
Statistical analyses were performed by using MATLAB (R2018b, MathWorks, USA). Mean, standard error of mean, minima and maxima of the CH4 flux values were calculated as common statistical parameters. Normality of the distribution of CH4 fluxes was tested with the Kolmogorov–Smirnov test, and due to non-normal distributions, non-parametric tests were used. The differences of medians from zero were tested with sign test. Difference between the day and night fluxes was tested with the Mann–Whitney U-test. Correlations between the CH4 fluxes and the environmental variables were tested using the Spearman´s rank correlation. Further analysis of correlation between the sap flow and the CH4 flux was performed by comparing the sap flow to the residuals of fitted model between the soil temperature and stem CH4 flux. Significance was assessed with a limit of p < 0.05 in all statistical tests.
Results
Environmental conditions
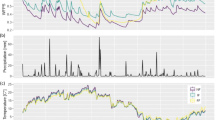
Over the measurement campaign (from 28 April to 11 June) the daily mean air temperature had an increasing trend from 9 to 17 May, while the increment seemed to pause in the middle of the season (Fig. 1). This is also the time when heavier precipitation/rainfall were recorded (Fig. 1). The cumulative precipitation and the mean air temperature over the measurement campaign of 8 weeks were 108.6 mm and 8.7 °C, respectively. The thermal spring turned into thermal summer on 25.5.2015 (Finnish Meteorological Institute (FMI) n.d.).
Methane exchange of stems, shoots and forest floor
During the measurement campaign we observed both emissions and occasional uptake of CH4 by the stems and shoots of birch and spruce. The mean and median fluxes calculated over the measurement period indicated mainly emissions from the stems and shoots of both species at both sites. At the forested fen, the CH4 fluxes of the birch and spruce stems ranged from − 1.3 to 430 and from − 1.2 to 6.5 µg m−2 h−1 (of stem surface area), respectively, and the median CH4 emissions from the birch stems (0.11–100 µg m−2 h−1, depending on the height) were mainly higher than from spruces (− 0.28–0.74 µg m−2 h−1) (Table 3). Regarding the birches at the fen, the emissions from the height of 0.5 m were substantially higher (medians 51 and 100 µg m−2 h−1, for birches n. 1 and 2 respectively; Table 3) than from the upper parts of the stems (medians 0.11–0.64 µg m−2 h−1; Table 3), and increased as the growing season proceeded (Fig. 2a). The spruce stems did not show such clear pattern regarding stem height (Table 3) nor temporal variation (Fig. 2b). At the stem height of 2.9 m, CH4 uptake by spruce dominated CH4 emission (Table 3). In contrast to the birches at the forested fen site, the stem CH4 fluxes of the birch tree at the upland site varied from − 1.3 to 11 µg m−2 h−1 (Table 3), and the fluxes did not show clear temporal or height-related patterns (Fig. 2c)
Stem CH4 fluxes (µg CH4 m–2 h–1) of (a) two downy birches (B. pubescens) and (b) two Norway spruces (P. abies) growing at the forested fen site, and (c) one downy birch (B. pubescens) growing at the upland forest site, and soil CH4 fluxes (d) at forested fen and upland site. All fluxes were measured in April–June 2015. The symbols in stem flux figures (a,b,c) are highlighted black, grey and white denoting the measurement height of 0.3–0.5 m, 2.9–3.6 m and 5.9–7.3 m of the tree stems, respectively. In soil CH4 flux figure (d), Fen1, Fen2, and Fen3 denote manual chambers at the forested fen site, and Upland denotes semi-automatic chamber at the upland site. Flux values inside the grey area were below the method quantification limit. Notice the differing y-axis scales
The CH4 fluxes from the birch shoots at the forested fen indicated mean emissions of 13 and 63 µg m−2 h−1 of leaf area from birches 1 and 2, respectively, while the mean shoot fluxes of two spruces showed both emission and uptake (24 and − 6.6 µg m−2 h−1 from spruces 3 and 4, respectively) (Table 3). The median flux of the fen birch no. 2 (4.6 µg m−2 h−1) was significantly different from zero (p < 0.05), while the other trees indicated small median emissions (0.45–0.88 µg m−2 h−1). The shoot CH4 fluxes did not indicate any seasonal dynamics related to leaf growth.
We found a positive correlation between the soil temperature and the CH4 flux from the near-ground stem parts of the birch trees at the forested fen site (rs = 0.61, p < 0.05, and rs = 0.92, p < 0.0001, for the two birches separately; soil temperature at 0.15–0.20 m; Fig. 3). The correlations were only slightly weaker with the soil temperature closer to the surface (rs = 0.52, p < 0.05; rs = 0.82, p < 0.001; data not shown). Otherwise, tree stem fluxes or shoot fluxes did not correlate significantly with soil temperature or with soil water content, soil CH4 fluxes, sap flow, air temperature, or radiation (p > 0.05). We did not observe any difference between the medians of the daytime and the night-time CH4 fluxes, neither for the stems or shoots, nor the forest floor (night-time medians: 0.62 µg m−2 h−1 (stems), 0.0025 µg m−2 h−1 (shoots), 110 µg m−2 h−1 (soil)).
The forest floor at the forested fen site was a net source of CH4 with a range from − 3.5 µg m−2 h−1 to 210 µg m−2 h−1, and a mean of 48 µg m−2 h−1 (Table 3). In contrast, the forest floor fluxes measured at the upland site showed CH4 uptake varying from − 180 to − 13 µg m−2 h−1 with a mean value of − 140 µg m−2 h−1 (Table 3). Only one of the three soil chambers at the forested fen site showed seasonal increase in the CH4 emissions from April to May, while in other locations at the forested fen and at the upland site the forest floor fluxes remained rather constant (Fig. 2d).
Upscaling of the stem and shoot fluxes at the fen site
The upscaled CH4 fluxes show that the studied trees were net emitters of CH4. The upscaled flux values (mean ± SEM) for the stems of birch and spruce were 14 ± 4.0 and 0.62 ± 0.20 mg ha−1 h−1, and for the shoots of birch and spruce 3.5 ± 1.7 and 127 ± 97 mg ha−1 h−1, respectively (Fig. 4). The sum of stem and canopy CH4 emissions were 17 ± 5.7 mg ha−1 h−1 from birch trees and 128 ± 97 mg ha−1 h−1 from spruce trees, respectively. The forest floor emitted 500 ± 100 mg ha−1 h−1 CH4. The combined CH4 emission rate of the trees and forest floor was 650 ± 200 mg ha−1 h−1, where trees represent 22% of the total flux.
Abundances of methanogenic and methanotrophic microbes
The quantification of the methanogenic mcrA genes was successful (abundance above the quantification limit = 101 gene copies reaction−1) only for the peat samples of the forested fen site, where the gene abundances varied between 2.6 × 107 and 2.3 × 108 copies g−1 (DW) within the two sampled years (Table 4). For the methanotrophic pmoA genes, the quantification was successful at the forested fen site for the peat samples (gene abundances between 3.7 × 107 and 4.6 × 108 copies g−1 DW), and for the roots of E. sylvaticum, S. girgensohnii and Salix sp., which had gene abundances between 2.3 × 105 and 6.6 × 107 copies g−1 (DW). At the upland site, pmoA abundances were quantifiable for the humus and litter samples, and for the P. sylvestris roots (between 4.4. × 105 and 3.4 × 107 copies g−1 DW). In addition, one of the two deadwood samples collected from the upland site had a pmoA abundance of 3.8 × 105 copies g−1 (DW). The values represent mean abundances, while the standard errors are given in Table 4 when applicable (n = 1 for 2014 and n = 2 to 3 for 2015). None of the above sample types differed significantly from each other (only applicable for the 2015 samples). The analysed samples, in which these genes could not be quantified, are listed in Supporting Information (Table S1).
Discussion
We studied the CH4 exchange of common boreal trees and the effect of spring leaf-out to the fluxes. This study complements the scarce research on boreal tree CH4 exchange, and especially globally rare shoot flux measurements (Machacova et al. 2016). Surprisingly, we did not observe any effect of spring recovery or leaf-out to the shoot CH4 exchange. Furthermore, there was no clear temporal pattern in the stem CH4 exchange, except for the bottom parts of the birch stems at the fen site. In addition, the CH4 exchange of neither stems nor shoots correlated with the sap flow.
Highly variable stem CH 4 emissions
In this study, measured trees showed highly variable stem CH4 dynamics both in time between the measurement days and between tree species. The emissions from birch stems at the fen site followed the pattern of decreasing CH4 emissions with stem height (Barba et al. 2019a), similar to earlier studies in temperate wetland and upland forests (Pangala et al. 2015; Wang et al. 2016; Jeffrey et al. 2020b; Schindler et al. 2020). The spruces at the fen and the birch at the upland site also emitted CH4 from their stems, but in much smaller amounts and without a similar height-related pattern than the fen birches.
Overall, the CH4 emissions from the tree stems in this study (medians between − 0.28–100 µg m−2 h−1, depending on the species, height and site) were rather small, yet in the same magnitude, compared to the flux rates reported from the tree stems in temperate zones (Terazawa et al. 2007, 2015; Pangala et al. 2015; Pitz and Megonigal 2017; Maier et al. 2018; Pitz et al. 2018; Barba et al. 2019b; Moldaschl et al. 2021). Pangala et al. (2015) reported an average summertime CH4 emission of 203 ± 21 µg m−2 h−1 from downy birch stems. In our study, the measurement period was limited to the spring and early summer, and thus we were not able to see if the emissions of birches continued to increase during the summer. Some previous studies reported substantial variety in the stem CH4 exchange between tree individuals (Terazawa et al. 2015; Maier et al. 2018; Moldaschl et al. 2021; Köhn et al. 2021), similarly as the fen birches in this study.
Sources of the stem-emitted CH4
Stem-emitted CH4 has been proposed to originate either (1) from microbial production in the soil, where CH4 is then taken up by tree roots, transported to the aboveground tissues and released to the atmosphere (Rusch and Rennenberg 1998), or (2) from in situ CH4 production by microorganisms inside the tree stems (Zeikus and Ward 1974; Covey et al. 2012; Yip et al. 2019; Li et al. 2020). In our study, the CH4 emissions from the birch stems followed soil temperature, whereas no such correlation was found with the spruce trees. Similarly, Pangala et al. (2015) reported stem CH4 emissions of downy birch increasing from April to June. Several other studies have reported similar relation between the stem fluxes and soil temperature, suggesting that the CH4 originates from methanogenic activity within the soil (Pangala et al. 2013, 2015; Terazawa et al. 2015). We found high abundances of both methanogens and methanotrophs in the peat profile at the fen site, demonstrating potential CH4 production and oxidation in the peat profile. Forest floor CH4 fluxes were close to zero in two out of three soil chambers (Fig. 2d), presumably also linked to methanotrophic activity at the peat surface (Putkinen et al. 2012). Interestingly, simultaneous CH4 fluxes from especially birch stems were high indicating that birches act as conduits for the soil-CH4, hence bypassing the peat profile where CH4 could be oxidized by methanotrophs. The forest floor CH4 fluxes at the forested fen site were in line with previous reports from pristine spruce mires (Huttunen et al. 2003; Koskinen et al. 2016).
Pangala et al. (2014) and Maier et al. (2018) found that the amount of CH4 dissolved in soil pore water was controlling the rate of stem CH4 flux of black alder (Alnus glutinosa) saplings and mature European beech (Fagus sylvatica), whereas Machacova et al. (2016) found significant correlations between CH4 fluxes of soil and tree stems of mature Scots pine. These studies suggest that soil CH4 concentration is a critically important driver of tree stem CH4 emission, however, none of the studies confirmed the CH4 transport mechanism. The soil-borne CH4 is suggested to be transported into the atmosphere either passively by diffusion via aerenchymatic structures and/or intercellular spaces (Machacova et al. 2013; Pangala et al. 2014; Terazawa et al. 2015; Maier et al. 2018; Plain & Epron 2021), or actively via transpiration stream of the trees (Pangala et al. 2015; Machacova et al. 2016).
Even though there was no correlation between the stem CH4 exchange and the sap flow nor between stem and soil CH4 fluxes in our study, this does not rule out the possibility that CH4 is transported in stems via transpiration stream. Recent modelling study by Anttila et al. (submitted manuscript) states that the transpiration stream is in fact the major transport pathway of axial CH4 movement in tree stems, however, due to the long transit time of CH4 within the stem and the resulting CH4 storage in the stems, the temporal dynamics of sap flow are not visible in the stem CH4 fluxes. Further, higher sap flow rate in birches (Gartner et al. 2009) could result a greater CH4 storage within birch stems and partly explain higher stem CH4 fluxes from the birch stems. Similar to our study, Schindler et al. (2021) did not detect any clear differences in stem CH4 emissions of Alnus incana between the daytime and night-time. However, diurnal measurement of CH4 fluxes from stems and shoots are scarce and should be considered in future work as omitting them might lead to over- or underestimation of upscaled CH4 fluxes.
All the sample trees at the forested fen site are growing under similar conditions under high water table, and thus the differences in CH4 exchange between the studied species may be due to different in situ CH4 production mechanisms, or differences in the physiology and/or anatomy of the species. One such difference is the root development: the roots of downy birch at peatland sites grow both in vertical and horizontal directions (Huikari 1959), whereas Norway spruce is traditionally assumed to grow roots closer the soil surface (e.g. Puhe 2003; Konôpka et al. 2010). Thus, possibly only the birch roots were penetrating into the deeper soil layers that are conductive to CH4 production. The angiosperms (including broad-leaved trees) are noted to cope better with waterlogging than gymnosperms (including conifers) (Kozlowski and Pallardy 1997). The primary adaptation of plants to flooding is the capacity to transport O2 from the atmosphere into the roots (Kozlowski 1997) through structures such as hypertrophied lenticels, aerenchymatic tissue in the roots and/or adventitious roots (Hook 1984). It is, however, yet to be confirmed whether this pathway is potential either for downy birch or Norway spruce.
While CH4 emissions at the fen site, especially by the birches, were presumably caused by the transportation of soil-derived CH4, the origin of the emitted CH4 from the birch at the upland site remains unclear. It is possible that methanogens inhabit upland soils (Angel et al. 2012; Lyu and Lu 2018) and we have earlier (in 2012) measured mcrA gene copies between 1.1 × 105 and 3.6 × 106 copies g−1 (DW) at moist regimes of the upland site (unpublished results) to which roots can have access. Recently, Barba and colleagues (Barba et al. 2021) reported elevated CH4 concentrations and significant CH4 emissions from upland trees growing on soils with below ambient CH4 concentrations. This supports earlier findings that even upland trees may have CH4 production within living stems, and this CH4 production has been connected to methanogenic archaea living in wet and/or rotten heartwood (Yip et al. 2019; Li et al. 2020). While we could not sample the heartwood of the same trees from which we measured the fluxes, the lack of quantifiable mcrA genes in the comparable tree samples (Table 4 and S1) suggests that stem-inhabiting methanogens are a negligible CH4 source in this boreal forest. However, we cannot completely rule out the effect of other relevant microbes not covered by the mcrA gene-based approach, or the presence of populations below the quantification limits (Putkinen et al. 2021).
Canopy CH4 emissions affect the whole-tree CH4 budget
All the studied tree species emitted small amounts of CH4 from their shoots, but also occasional CH4 uptake was observed. These findings are in line with previously reported canopy CH4 exchange rates from boreal trees in Finland (Machacova et al. 2016) and in Sweden (Sundqvist et al. 2012; Putkinen et al. 2021). When the net emissions on the forested fen site were upscaled per canopy biomass of the trees, the total canopy emissions were nine-fold compared to tree stem emissions in trees, due to high leaf biomass. This indicate that even small emissions at the leaf-level may have a substantial contribution to the whole-tree CH4 dynamics, and to the ecosystem CH4 budget during the spring leaf-out period. As the measured shoot fluxes in both birch and spruce canopies were very small and highly variable, our findings should be considered as indicative. The used measurement technique was able to detect CH4 fluxes even with a relatively long chamber closure time, which certainly affected the physiological functioning of the tree. Excessive heating of the chamber was avoided by physical shading of the chambers, which blocked the incoming UV- and photosynthetically active radiation (PAR) to the leaves. As a result, the possible light-driven CH4 emissions, found in laboratory studies (Keppler et al. 2006; Vigano et al. 2008; Fraser et al. 2015; Martel and Qaderi 2019), were likely missing. This could also explain why we did not find a connection between the shoot CH4 emissions and radiation (PAR, UV). The high variability in CH4 exchange by the shoots also suggests that both uptake and emission may be present as reported also by Sundqvist et al. (2012) and Putkinen et al. (2021).
The leaf-level CH4 consumption detected in the previous field studies (Sundqvist et al. 2012; Putkinen et al. 2021) was hypothesized to result from methanotrophic activity in the leaves, while the presence of currently recognized methanotrophs was not confirmed in their studied trees. In our study, we were unable to detect quantifiable amount of methanotrophs in the analysed shoot samples. Still, lack of quantification does not rule out small populations, which is supported by previous detection of variable alphaproteobacterial methanotrophs in conifer needles by cultivation (Doronina et al. 2004; Iguchi et al. 2012) and 16S rRNA sequencing methods (Rúa et al. 2016; Haas et al. 2018). In the seasonal follow-up by Haas et al. (2018), needle methanotroph abundance increased towards the end of the growing season – a pattern, which could partly explain our results from the early summer. In addition, the methanotroph detected by Haas et al. (2018) was of the genus Methylocella, which lacks the functional pmoA gene targeted in our study.
Upscaling the forest floor, tree stem and canopy fluxes to the whole-forest exchange at the forested fen, we found that the trees contributed significantly to the net CH4 balance. Inclusion of the CH4 emissions from the tree stems and shoots increased the total CH4 emissions by 29% compared to the forest floor flux of the site. Similar whole-forest upscaling, including stem and canopy CH4 exchange, has only been conducted by Machacova et al. (2016) for Scots pine trees at an adjacent experimental upland forest plot at Hyytiälä. In that upscaling, the CH4 emissions from Scots pine trees offset 0.8% of the CH4 sink of the upland soil. Our results indicate that at the forested fen, where the soil is a significant source of CH4, a large part of the ecosystem CH4 emissions could still originate from trees. The canopy-scale CH4 emissions, however, should be considered with caution due to the high uncertainties in the measured fluxes as well as in the methods to upscale to the ecosystem level. Furthermore, the number of studied trees was very limited. The upscaling here needs to be considered as one of the first efforts to understand the role of tree canopies and stems to the whole-forest CH4 balance, and more measurements with higher temporal coverage and with an improved canopy flux measurement method are required for reliable estimations.
According to this study, trees at the boreal vegetation zone are capable of emitting CH4 from their stems and canopies, with the emission rates in the same magnitude as those from temperate forests. The variation in stem CH4 flux between tree species, tree individuals and different habitats is high, and the CH4 emissions from high-emitting trees – birches on peat soil – increase as the growing season proceeds. The lack of detection of methanogens or methanotrophs in the aboveground plant tissues suggests that these microbes did not have a significant role in the observed tree-derived CH4 fluxes. At least at the fen site, the stem-emitted CH4 from birch trees is most likely produced microbially in the soil. More research is needed to study the possible differences between tree species. Long-term and continuous CH4 flux measurements of tree stems and shoots are critically needed to accurately identify the drivers behind the CH4 dynamics and to assess the contribution of trees on forest CH4 budget. Finally, studies regarding trees part taking in CH4 dynamics are critically important for creating a comprehensive baseline data to be used in research of climate change and its drivers.
References
Althoff F, Benzing K, Comba P et al (2014) Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat Commun 5:4205. https://doi.org/10.1038/ncomms5205
Angel R, Claus P, Conrad R (2012) Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J 6:847–862. https://doi.org/10.1038/ismej.2011.141
Barba J, Bradford MA, Brewer PE et al (2019a) Methane emissions from tree stems: a new frontier in the global carbon cycle. New Phytol 222:18–28. https://doi.org/10.1111/nph.15582
Barba J, Poyatos R, Capooci M, Vargas R (2021) Spatiotemporal variability and origin of CO2 and CH4 tree stem fluxes in an upland forest. Glob Chang Biol 27:4879–4893. https://doi.org/10.1111/gcb.15783
Barba J, Poyatos R, Vargas R (2019b) Automated measurements of greenhouse gases fluxes from tree stems and soils: magnitudes, patterns and drivers. Sci Rep 9:4005. https://doi.org/10.1038/s41598-019-39663-8
Bender M, Conrad R (1993) Kinetics of methane oxidation in oxic soils. Chemosphere 26:687–696. https://doi.org/10.1016/0045-6535(93)90453-c
Benzing K, Comba P, Martin B et al (2017) Nonheme Iron-Oxo-Catalyzed Methane Formation from Methyl Thioethers: Scope, Mechanism, and Relevance for Natural Systems. Chem - A Eur J 23:10465–10472. https://doi.org/10.1002/chem.201701986
Bourne DG, McDonald IR, Murrell JC (2001) Comparison of pmoA PCR Primer Sets as Tools for Investigating Methanotroph Diversity in Three Danish Soils. Appl Environ Microbiol 67:3802–3809. https://doi.org/10.1128/AEM.67.9.3802-3809.2001
Carmichael MJ, Bernhardt ES, Bräuer SL, Smith WK (2014) The role of vegetation in methane flux to the atmosphere: should vegetation be included as a distinct category in the global methane budget? Biogeochemistry 119:1–24. https://doi.org/10.1007/s10533-014-9974-1
Conrad R (2009) The global methane cycle: Recent advances in understanding the microbial processes involved. Environ Microbiol Rep 1:285–292. https://doi.org/10.1111/j.1758-2229.2009.00038.x
Corley J (2003) Best practices in establishing detection and quantification limits for pesticide residues in foods. In: Lee PW, Aizawa H, Barefoot AC et al (eds) Handbook of Residue Analytical Methods for Agrochemicals. John Wiley & Sons Ltd, Chichester, pp 59–75
Covey KR, Wood SA, Warren RJ et al (2012) Elevated methane concentrations in trees of an upland forest. Geophys Res Lett 39:L15705. https://doi.org/10.1029/2012GL052361
Doronina NV, Ivanova EG, Suzina NE, Trotsenko YA (2004) Methanotrophs and methylobacteria are found in woody plant tissues within the winter period. Microbiology 73:702–709
Farhan Ul Haque M, Xu H-J, Colin Murrell J, Crombie A (2020) Facultative methanotrophs – diversity, genetics, molecular ecology and biotechnological potential: A mini-review. Microbiology 166:894–908
Finnish Meteorological Institute (FMI) (n.d.) Seasonal climate statistics. In: Kesän 2015 sää. Available via https://www.ilmatieteenlaitos.fi/kesa-2015
Fraser WT, Blei E, Fry SC et al (2015) Emission of methane, carbon monoxide, carbon dioxide and short-chain hydrocarbons from vegetation foliage under ultraviolet irradiation. Plant, Cell Environ 38:980–989. https://doi.org/10.1111/pce.12489
Gartner K, Nadezhdina N, Englisch M et al (2009) Sap flow of birch and Norway spruce during the European heat and drought in summer 2003. For Ecol Manage 258:590–599. https://doi.org/10.1016/j.foreco.2009.04.028
Gauci V, Gowing DJG, Hornibrook ERC et al (2010) Woody stem methane emission in mature wetland alder trees. Atmos Environ 44:2157–2160. https://doi.org/10.1016/j.atmosenv.2010.02.034
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol 3:309–320. https://doi.org/10.1093/treephys/3.4.309
Haas JC, Street NR, Sjödin A et al (2018) Microbial community response to growing season and plant nutrient optimisation in a boreal Norway spruce forest. Soil Biol Biochem 125:197–209. https://doi.org/10.1016/j.soilbio.2018.07.005
Halmeenmäki E, Heinonsalo J, Putkinen A et al (2017) Above- and belowground fluxes of methane from boreal dwarf shrubs and Pinus sylvestris seedlings. Plant Soil 420:361–373. https://doi.org/10.1007/s11104-017-3406-7
Hari P, Kulmala M (2005) Station for Measuring Ecosystem-Atmosphere Relations (SMEAR II). Boreal Environ Res 10:315–322
Hartmann DJ, Klein Tank AMG, Rusticucci M et al (2013) Observations: Atmosphere and Surface. In: Stocker TF, Qin D, Plattner G-K et al (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 159–254
Holland PW, Welsch RE (1977) Robust regression using iteratively reweighted least-squares. Commun Stat - Theory Methods 6:813–827. https://doi.org/10.1080/03610927708827533
Holmes AJ, Costello A, Lidstrom ME, Murrell JC (1995) Evidence that participate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132:203–208. https://doi.org/10.1111/j.1574-6968.1995.tb07834.x
Hölttä T, Kolari P (2009) Interpretation of stem CO2 efflux measurements. Tree Physiol 29:1447–1456. https://doi.org/10.1093/treephys/tpp073
Hook DD (1984) Adaptations to flooding with fresh water. In: Kozlowski TT (ed) Flooding and Plant Growth. Academic Press, Orlando, pp 265–294
Huikari O (1959) On the effect of anaerobic media upon the roots of birch, pine and spruce seedlings. Commun Instituti for Fenn 50:9
Huttunen JT, Nykänen H, Turunen J, Martikainen PJ (2003) Methane emissions from natural peatlands in the northern boreal zone in Finland, Fennoscandia. Atmos Environ 37:147–151. https://doi.org/10.1016/S1352-2310(02)00771-9
Iguchi H, Sato I, Sakakibara M et al (2012) Distribution of methanotrophs in the phyllosphere. Biosci Biotechnol Biochem 76:1580–1583. https://doi.org/10.1271/bbb.120281
Jeffrey LC, Maher DT, Tait DR et al (2020a) (2020a) Tree stem methane emissions from subtropical lowland forest (Melaleuca quinquenervia) regulated by local and seasonal hydrology. Biogeochemistry 151:273–290. https://doi.org/10.1007/s10533-020-00726-y
Jeffrey LC, Maher DT, Tait DR et al (2020b) A Small Nimble In Situ Fine-Scale Flux Method for Measuring Tree Stem Greenhouse Gas Emissions and Processes (S.N.I.F.F). Ecosystems 23:1676–1689. https://doi.org/10.1007/s10021-020-00496-6
Jeffrey LC, Maher DT, Chiri E et al (2021a) Bark-dwelling methanotrophic bacteria decrease methane emissions from trees. Nat Commun 12:2127. https://doi.org/10.1038/s41467-021-22333-7
Jeffrey LC, Maher DT, Tait DR et al (2021b) Isotopic evidence for axial tree stem methane oxidation within subtropical lowland forests. New Phytol 230:2200–2212. https://doi.org/10.1111/nph.17343
Junninen H, Lauri A, Keronen P et al (2009) Smart-SMEAR : on-line data exploration and visualization tool for SMEAR stations. Boreal Environ Res 14:447–457
Keppler F, Hamilton JTG, Braß M, Röckmann T (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature 439:187–191. https://doi.org/10.1038/nature04420
Keppler F, Hamilton JTG, McRoberts WC et al (2008) Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies. New Phytol 178:808–814. https://doi.org/10.1111/j.1469-8137.2008.02411.x
Köhn D, Günther A, Schwabe I, Jurasinski G (2021) Short-lived peaks of stem methane emissions from mature black alder (Alnus glutinosa (L.) Gaertn.) – Irrelevant for ecosystem methane budgets? Plant-Environment Interact 2:16–27. https://doi.org/10.1002/pei3.10037
Konôpka B, Moravčík M, Pajtík J, Lukac M (2010) Effect of soil waterlogging on below-ground biomass allometric relations in Norway spruce. Plant Biosyst 144:448–457. https://doi.org/10.1080/11263501003726391
Koskinen M, Maanavilja LM, Nieminen M, et al (2016) High methane emissions from restored Norway spruce swamps in southern Finland over one growing season. Mires Peat 17:2. https://doi.org/10.19189/MaP.2015.OMB.202
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol 17:490–490. https://doi.org/10.1093/treephys/17.7.490
Kozlowski TT, Pallardy SG (1997) Growth Control in Woody Plants. Academic Press, San Diego
Lenhart K, Althoff F, Greule M, Keppler F (2015) Technical note: Methionine, a precursor of methane in living plants. Biogeosciences 12:1907–1914. https://doi.org/10.5194/bg-12-1907-2015
Li H-L, Zhang X-M, Deng F-D et al (2020) Microbial methane production is affected by secondary metabolites in the heartwood of living trees in upland forests. Trees 34:243–254. https://doi.org/10.1007/s00468-019-01914-6
Livingston GP, Hutchinson GL (1995) Enclosure-based measurement of trace gas exchange: applications and sources of error. In: Matson PA, Lawton JH, Harriss RC, Likens GE (eds) Biogenic trace gases: Measuring emissions from soil and water. Blackwell Science, Oxford, pp 14–51
Lyu Z, Lu Y (2018) Metabolic shift at the class level sheds light on adaptation of methanogens to oxidative environments. ISME J 12:411–423. https://doi.org/10.1038/ismej.2017.173
Machacova K, Bäck J, Vanhatalo A et al (2016) Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci Rep 6:23410. https://doi.org/10.1038/srep23410
Machacova K, Maier M, Svobodova K et al (2017) Cryptogamic stem covers may contribute to nitrous oxide consumption by mature beech trees. Sci Rep 7:13243. https://doi.org/10.1038/s41598-017-13781-7
Machacova K, Papen H, Kreuzwieser J, Rennenberg H (2013) Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant Soil 364:287–301. https://doi.org/10.1007/s11104-012-1359-4
Machacova K, Vainio E, Urban O, Pihlatie M (2019) Seasonal dynamics of stem N2O exchange follow the physiological activity of boreal trees. Nat Commun 10:4989. https://doi.org/10.1038/s41467-019-12976-y
Maier M, Machacova K, Lang F et al (2018) Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J Plant Nutr Soil Sci 181:31–35. https://doi.org/10.1002/jpln.201600405
Mander Ü, Krasnova A, Schindler T et al (2021) Long-term dynamics of soil, tree stem and ecosystem methane fluxes in a riparian forest. Sci Total Environ 809. https://doi.org/10.1016/j.scitotenv.2021.151723
Martel AB, Qaderi MM (2019) Unravelling the effects of blue light on aerobic methane emissions from canola. J Plant Physiol 233:12–19. https://doi.org/10.1016/j.jplph.2018.12.006
Messenger DJ, McLeod AR, Fry SC (2009) The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ 32:1–9. https://doi.org/10.1111/j.1365-3040.2008.01892.x
Moldaschl E, Kitzler B, Machacova K et al (2021) Stem CH4 and N2O fluxes of Fraxinus excelsior and Populus alba trees along a flooding gradient. Plant Soil 461:407–420. https://doi.org/10.1007/s11104-020-04818-4
Pangala SR, Enrich-Prast A, Basso LS et al (2017) Large emissions from floodplain trees close the Amazon methane budget. Nature 552:230–234. https://doi.org/10.1038/nature24639
Pangala SR, Gowing DJ, Hornibrook ERC, Gauci V (2014) Controls on methane emissions from Alnus glutinosa saplings. New Phytol 201:887–896. https://doi.org/10.1111/nph.12561
Pangala SR, Hornibrook ERC, Gowing DJ, Gauci V (2015) The contribution of trees to ecosystem methane emissions in a temperate forested wetland. Glob Chang Biol 21:2642–2654. https://doi.org/10.1111/gcb.12891
Pangala SR, Moore S, Hornibrook ERC, Gauci V (2013) Trees are major conduits for methane egress from tropical forested wetlands. New Phytol 197:524–531. https://doi.org/10.1111/nph.12031
Pihlatie M, Christiansen JR, Aaltonen H et al (2013) Comparison of static chambers to measure CH4 emissions from soils. Agric for Meteorol 171–172:124–136. https://doi.org/10.1016/j.agrformet.2012.11.008
Pirinen P, Simola H, Aalto J et al (2012) Climatological statistics of Finland 1981–2010. Reports 2012:96
Pitz S, Megonigal JP (2017) Temperate forest methane sink diminished by tree emissions. New Phytol 214:1432–1439. https://doi.org/10.1111/nph.14559
Pitz SL, Megonigal JP, Chang C-H, Szlavecz K (2018) Methane fluxes from tree stems and soils along a habitat gradient. Biogeochemistry 137:307–320. https://doi.org/10.1007/s10533-017-0400-3
Plain C, Epron D (2021) Pulse labelling of deep soil layers in forest with 13CH4: testing a new method for tracing methane in the upper horizons, understorey vegetation and tree stems using laser-based spectrometry. Biogeochemistry 153:215–222. https://doi.org/10.1007/s10533-021-00775-x
Plain C, Ndiaye F-K, Bonnaud P et al (2019) Impact of vegetation on the methane budget of a temperate forest. New Phytol 221:1447–1456. https://doi.org/10.1111/nph.15452
Puhe J (2003) Growth and development of the root system of Norway spruce (Picea abies) in forest stands - A review. For Ecol Manage 175:253–273. https://doi.org/10.1016/S0378-1127(02)00134-2
Putkinen A, Larmola T, Tuomivirta T et al (2012) Water dispersal of methanotrophic bacteria maintains functional methane oxidation in sphagnum mosses. Front Microbiol 3:15. https://doi.org/10.3389/fmicb.2012.00015
Putkinen A, Siljanen HMP, Laihonen A, et al (2021) New insight to the role of microbes in the methane exchange in trees – evidence from metagenomic sequencing. New Phytol nph.17365. https://doi.org/10.1111/nph.17365
Repola J (2009) Biomass equations for Scots pine and Norway spruce in Finland. Silva Fenn 43:625–647. https://doi.org/10.14214/sf.184
Repola J (2008) Biomass equations for birch in Finland. Silva Fenn 42:605-624. https://doi.org/10.14214/sf.236
Rúa MA, Wilson EC, Steele S et al (2016) Associations between ectomycorrhizal fungi and bacterial needle endophytes in Pinus radiata: implications for biotic selection of microbial communities. Front Microbiol 7:399. https://doi.org/10.3389/fmicb.2016.00399
Rusch H, Rennenberg H (1998) Black alder (Alnus glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 201:1–7. https://doi.org/10.1023/A:1004331521059
Schindler T, Mander Ü, Machacova K, Espenberg M, Krasnov D, Escuer-Gatius J, Veber G, Pärn J, Soosaar K (2020) Short-term flooding increases CH4 and N2O emissions from trees in a riparian forest soil-stem continuum. Sci Rep 10:3204. https://doi.org/10.1038/s41598-020-60058-7
Schindler T, Machacova K, Mander Ü et al (2021) Diurnal Tree Stem CH4 and N2O Flux Dynamics from a Riparian Alder Forest. Forests 12(7):863. https://doi.org/10.3390/f12070863
Sjögersten S, Siegenthaler A, Lopez OR, Aplin P, Turner B, Gauci V (2020) Methane emissions from tree stems in neotropical peatlands. New Phytol 225:769–781. https://doi.org/10.1111/nph.16178
Steinberg LM, Regan JM (2008) Phylogenetic Comparison of the Methanogenic Communities from an Acidic, Oligotrophic Fen and an Anaerobic Digester Treating Municipal Wastewater Sludge. Appl Environ Microbiol 74:6663–6671. https://doi.org/10.1128/AEM.00553-08
Sundqvist E, Crill P, Mlder M et al (2012) Atmospheric methane removal by boreal plants. Geophys Res Lett 39:10–15. https://doi.org/10.1029/2012GL053592
Takahashi K, Kosugi Y, Kanazawa A, Sakabe A (2012) Automated closed-chamber measurements of methane fluxes from intact leaves and trunk of Japanese cypress. Atmos Environ 51:329–332. https://doi.org/10.1016/j.atmosenv.2012.01.033
Tenhovirta SAM, Kohl L, Koskinen M, Patama M, Lintunen A, Zanetti A, Lilja R and Pihlatie M (2022) Solar radiation drives methane emissions from the shoots of Scots pine. New Phytologist. Accepted Author Manuscript. https://doi.org/10.1111/nph.18120
Terazawa K, Ishizuka S, Sakata T et al (2007) Methane emissions from stems of Fraxinus mandshurica var. japonica trees in a floodplain forest. Soil Biol Biochem 39:2689–2692. https://doi.org/10.1016/j.soilbio.2007.05.013
Terazawa K, Yamada K, Ohno Y et al (2015) Spatial and temporal variability in methane emissions from tree stems of Fraxinus mandshurica in a cool-temperate floodplain forest. Biogeochemistry 123:349–362. https://doi.org/10.1007/s10533-015-0070-y
Timonen S, Sinkko H, Sun H et al (2017) Ericoid Roots and Mycospheres Govern Plant-Specific Bacterial Communities in Boreal Forest Humus. Microb Ecol 73:939–953. https://doi.org/10.1007/s00248-016-0922-6
Vainio E, Peltola O, Kasurinen V et al (2021) Topography-based statistical modelling reveals high spatial variability and seasonal emission patches in forest floor methane flux. Biogeosciences 18:2003–2025. https://doi.org/10.5194/bg-18-2003-2021
Vigano I, van Weelden H, Holzinger R et al (2008) Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosciences 5:937–947. https://doi.org/10.5194/bg-5-937-2008
Wang ZP, Gu Q, Deng FD et al (2016) Methane emissions from the trunks of living trees on upland soils. New Phytol 211:429–439. https://doi.org/10.1111/nph.13909
Xu X, Yuan F, Hanson PJ et al (2016) Reviews and syntheses: Four decades of modeling methane cycling in terrestrial ecosystems. Biogeosciences 13:3735–3755. https://doi.org/10.5194/bg-13-3735-2016
Yip DZ, Veach AM, Yang ZK et al (2019) Methanogenic Archaea dominate mature heartwood habitats of Eastern Cottonwood (Populus deltoides). New Phytol 222:115–121. https://doi.org/10.1111/nph.15346
Zeikus JG, Ward JC (1974) Methane formation in living trees: a microbial origin. Science 184:1181–1183. https://doi.org/10.1126/science.184.4142.1181
Acknowledgements
This work was supported by the Emil Aaltonen Foundation, Academy of Finland (138041, 272091, 292699, 294088, 288494), The Academy of Finland Centre of Excellence (272041), ICOS-FINLAND (281255), Czech Science Foundation (17-18112Y), project ENVIMET (CZ.1.07/2.3.00/20.0246), the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Program I (grant number LO1415), project SustES – Adaptation strategies for sustainable ecosystem services and food security under adverse environmental conditions (CZ.02.1.01/0.0/0.0/16_019/0000797), and from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under grant agreement No [757695]. We thank the staff of SMEAR II for technical assistance, and Teemu Paljakka for the sap flow data.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Contributions
M.P. and K.M. had the original idea of the study. M.P. and K.M. designed the study. I.H., E.V., K.M., and M.P. carried out the field measurements. I.H. and E.V. analysed the flux data. A.P., T.T., H.F. and M.S. carried out the laboratory work and analysed the microbial data. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have financial or non-financial conflicting interests related to the work submitted for publication.
Additional information
Responsible Editor: Stefan K. Arndt.
Elisa Vainio and Iikka P. Haikarainen contributed equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vainio, E., Haikarainen, I.P., Machacova, K. et al. Soil-tree-atmosphere CH4 flux dynamics of boreal birch and spruce trees during spring leaf-out. Plant Soil 478, 391–407 (2022). https://doi.org/10.1007/s11104-022-05447-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05447-9