Abstract
Cu+-doped lithium aluminates with LiAlO2, LiAl5O8, and Li5AlO4 stoichiometry were investigated. Tetragonal γ-LiAlO2 and cubic α-LiAl5O8 were prepared in the form of bulk ceramics using the Pechini sol–gel route with Cu-doping concentrations up to 1 mol%. For γ-LiAlO2, single-phase samples were obtained only for the lowest doping concentration tested, which was 0.1 mol%, while α-LiAl5O8 samples were single phase within the full concentration series. Radioluminescence and photoluminescence of Cu-doped γ-LiAlO2 featured only the transition from 3d9 4s1 to 3d10 corresponding to Cu+ ions. On the other hand, Cu-doped α-LiAl5O8 samples showed mainly emissions associated with Fe3+ and Cr3+ impurities with overall very small intensity compared to the reference BGO scintillator. Samples of Cu-doped β-Li5AlO4 were prepared using a nitrates decomposition method. Due to the volatility of Li2O, the samples were not in a single phase and contained a certain amount of secondary γ-LiAlO2 phase, even though excess lithium was used for the sample preparation. Furthermore, samples with β-Li5AlO4 exhibited strong hygroscopic properties, making Li5AlO4 an undesirable host material outside of a controlled atmosphere environment.

The graphical abstract shows the unit cells of the three lithium aluminate hosts investigated in this work, along with X-ray excited radioluminescence spectra obtained by doping copper into the γ-LiAlO2 and α-LiAl5O8 hosts.
Highlights
-
Single-phase samples of copper-doped γ-LiAlO2 and α-LiAl5O8 were obtained by the sol–gel method.
-
The Cu:LiAlO2 samples showed a single emission band, caused by a radiative transition of Cu+ ions.
-
The Cu:LiAl5O8 samples exhibited mainly low-intensity emission of undesired impurities.
-
The Cu:Li5AlO4 samples contained a secondary γ-LiAlO2 phase and were highly hygroscopic.
Similar content being viewed by others
1 Introduction
Detection of slow thermal neutrons utilizes a nuclear reaction, during which a neutron is captured by an isotope with a high thermal neutron cross-section, and high-energy charged particles are produced as a result. Historically, the isotope 3He was used for this purpose in the first proportional counter neutron detectors [1]. However, because of the low abundance of this isotope along with the increasing demand for neutron detectors in security, research, and other advanced fields, novel scintillation detectors have been explored as an alternative for neutron detection. Neutron scintillators can utilize neutron capture by other isotopes with high thermal neutron cross-sections, such as 6Li or 10B. High-energy charged particles produced in such a nuclear reaction then cause the emission of scintillation light along their ionization tracks [2, 3].
Various 6Li-based neutron scintillators have been investigated, such as 6Li-loaded glass [4], LiI [5, 6], or LiAlO2 [7, 8], all of which use the nuclear reaction 6Li(n,α)3H with a thermal neutron cross-section of 940 barn and a Q-value of 4.78 MeV [9]. LiAlO2 and lithium aluminates in general are suitable materials for neutron scintillation due to their high lithium content in addition to their low density and low atomic number. These qualities are favorable for minimizing the effectivity of the neutron scintillator toward high-energy photons (gamma rays). Depending on the lithium content, three different phases can be distinguished within the Li2O-Al2O3 system, the lithium-rich Li5AlO4, the intermediate LiAlO2, and the aluminum-rich LiAl5O8. The intermediate LiAlO2 phase has been studied as a tritium breeding blanket for thermonuclear fusion reactors [10, 11], a ceramic filler for lithium-ion polymer batteries [12], and also as an electrolyte matrix for molten carbon fuel cells [13]. Doped with transition metals or rare-earth elements, it has been investigated more recently as an optical material and a neutron scintillator in particular [14,15,16,17]. It can exist in three different polymorphs, low-temperature α-LiAlO2, high-pressure β-LiAlO2, and high-temperature γ-LiAlO2. The low-temperature α-LiAlO2 undergoes an irreversible transformation to γ-LiAlO2 at a temperature greater than 750 °C [18], while the formation of the β-LiAlO2 was not observed at atmospheric pressure [19]. Therefore, the main polymorph considered for scintillation applications is the tetragonal γ-LiAlO2. The aluminum-rich LiAl5O8 phase has also been studied as a host matrix in the development of luminescent materials activated by transition metal ions [20,21,22] or rare-earth ions [23,24,25,26]. LiAl5O8 crystallizes in a cubic lattice with an inverse spinel structure, although the existence of two forms with different unit cell symmetry has been described, that is, a low-temperature α-form with lower-symmetry primitive cubic cell and a high-temperature β-form with higher-symmetry face-centered cubic cell [27,28,29]. The lower symmetry of the α-form is caused by a 1:3 ordering of Li+ and Al3+ ions occupying the octahedral sites, while the remaining Al3+ ions are tetrahedrally coordinated. Above 1295 °C the ordered α-form is transformed into the disordered β-form, which no longer features any long-range ordering of Li+ and Al3+ ions occupying the octahedral sites [30]. The main form of LiAl5O8 reported in the literature for the research and development of luminescent materials is the low-temperature α-form. By far the least explored is the lithium-rich Li5AlO4 phase. Only a handful of publications reported the preparation of this phase, mainly through the solid-state synthesis in an undoped form for CO2 absorption [31, 32], electrical applications [33, 34], or as basic research [35, 36]. Two polymorphs were identified for Li5AlO4, a low-temperature α-phase, and a high-temperature β-phase. Both of these polymorphs are orthorhombic and undergo a phase transition from α to β at approximately 780 °C [37].
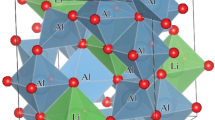
In this work, we focus on the preparation and luminescence characterization of the copper-doped lithium aluminate phases γ-LiAlO2, α-LiAl5O8, and β-Li5AlO4. Li+ and Cu+ ions have almost identical ionic radii and Cu+ ions can fulfill the role of a luminescence center in scintillators [38]. Figure 1 shows the crystal structure of the studied oxides; the figure caption summarizes the space group, density, and effective atomic number of depicted polymorphs. For Cu:LiAlO2 and Cu:LiAl5O8, series of samples were prepared using the Pechini sol–gel route with doping up to 1 mol% (from the position of lithium). The selected concentration range was based on the literature survey of optimal doping concentrations for different transition metals doped into the γ-LiAlO2 and α-LiAl5O8 hosts. In the case of copper-doped β-Li5AlO4 samples, the sol–gel preparation resulted mainly in the formation of highly stable Li2CO3, which is most likely related to large carbon contamination in samples from the carbon-based gel network. Therefore, we proposed a nitrates decomposition method for the preparation of copper-doped β-Li5AlO4, which consists of obtaining a solid mixture of metal nitrates followed by a solid-state reaction. Using this approach, we were able to obtain samples of copper-doped β-Li5AlO4 with a certain amount of γ-LiAlO2 secondary phase.
Unit cells of lithium aluminates within the Li2O-Al2O3 system: a β-Li5AlO4 (orthorombic, Pmmn, 2.17 g/cm3, Zeff 9.1) [44], b γ-LiAlO2 (tetragonal, P41212, 2.53 g/cm3, Zeff 10.7) [45], and c α-LiAl5O8 (cubic, P4332, 3.52 g/cm3, Zeff 11.0) [46]. Images were generated using the VESTA software [47] (lithium, aluminum, and oxygen atoms are shown in green, gray, and red, respectively)
2 Experimental
2.1 Sample preparation
The Pechini sol–gel route was used for all three lithium aluminates. The following chemicals were used for the synthesis: Al(NO3)3 ∙ 9H2O (Fischer Scientific; 99.999%), CH3COOLi (Strem; 98%), Cu(CH3COO)2 (Strem; 98%), citric acid (CA) (Lach-Ner; p.a.), ethylene glycol (EG) (Lach-Ner; p.a.). Aluminum nitrate was dissolved in distilled water and a stoichiometric amount of lithium acetate and copper acetate was added. Then CA and EG were added with a molar ratio of metals, CA, and EG of 1:4:16. The solution was heated at 90 °C until the water evaporated. Finally, the temperature was raised to 130 °C to reach gelation. The gel was dried at 250 °C for 2 h in the air. The xerogel was thermally decomposed at 500 °C for 2 h in the air with a ramp of 3 h. The precursor obtained was minced to a fine powder and further annealed at 800 °C for 2 h in the air. This powder was then pressed into 2 × 2 × 10 mm bars and annealed at the final annealing temperature, which varied according to the stoichiometry of the lithium aluminate host. The annealing atmosphere of this final annealing treatment was air, N2, or H2.
Furthermore, nitrates decomposition route was also used for the preparation of Cu-doped Li5AlO4 samples. In this case, aluminum nitrate was dissolved in water and then lithium acetate was added to the solution along with copper acetate. The lithium acetate was used in a stoichiometric amount or in excess to try to compensate for any Li evaporation in the later stages of the preparation. The Li:Al ratio in the prepared starting solution was approximately 5:1, 6:1, or 7.5:1, which corresponds to the use of the stoichiometric amount, 20% or 50% surplus of lithium acetate, respectively. The amount of copper acetate used corresponded to 0.1 mol% doping (substitution at the lithium site). In the next step, the starting aqueous solution was evaporated while constantly stirring and dried in air at 120 °C overnight. The resulting precursor was minced into a fine powder and then annealed in air at 1000 °C for 4 h. The compositions and conditions for the preparation of copper-doped lithium aluminate samples are summarized in Table 1.
2.2 Characterization
X-ray powder diffraction (XRD) data were collected at room temperature using a Bruker AXS D2 Phaser powder diffractometer with Bragg–Brentano parafocusing geometry using CoKα radiation (30 kV, 10 mA, 0.179021 nm).
Differential thermal and thermogravimetric (DTA/TG) analyses were carried out simultaneously using Setsys Evolution (Setaram) equipment. The temperature range of the measurement was 20–1000 °C with a heating rate of 10 °C/min; the measurement was carried out in an ambient atmosphere.
Photoluminescence properties were measured on a HORIBA Jobin Yvon Fluorolog®-3 Extreme spectrometer using FluorEssence™ 3 software. A photomultiplier tube with thermoelectric cooling and a Ce:InGaAs photocathode (detection range 185–900 nm) were used. Sample excitation was performed using a 450 W xenon continuous-wave lamp. For the spectra evaluation, all of the measured luminescence spectra were transformed to the base level and, after the subtraction of the background, normalized with the help of the reference sample (a Ce:YAG single crystal).
The radioluminescence spectra were measured at room temperature using a custom-made 5000 M Horiba Jobin Yvon fluorescence spectrometer with a TBX-04 photon-counting detector (IBH Scotland). The samples were excited by an X-ray tube (40 kV, 15 mA; Seifert Gmbh). All spectra were corrected for spectral distortions due to the setup. Powdered Bi4Ge3O12 (BGO) crystal was also measured as a reference scintillator.
Photoluminescence decay measurements were carried out by a 5000 M Horiba Jobin Yvon fluorometer using the time-correlated single photon counting technique. A microsecond xenon flashlamp and a nanoLED source with a typical FWHM below 2 ns (IHM Scotland) were used for excitation.
3 Results and discussion
3.1 Cu:LiAlO2
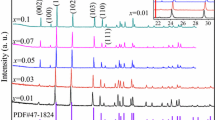
Figure 2 shows the XRD patterns of Cu-doped LiAlO2 ceramics. At the lowest doping concentration, the sample was a single phase with all reflections corresponding to the tetragonal γ-LiAlO2 phase, indexed according to PDF 04-009-6438. However, in the case of samples with higher doping concentrations of 0.5 or 1.0 mol%, the formation of a secondary CuO phase was observed, while no complementary Al2O3 phase was detected. This may be due to the lower response of aluminum atoms to X-rays compared to heavier copper atoms, causing the Al2O3 diffraction intensity to lie below the detection limit. However, only small concentrations of copper ions can be introduced into the tetragonal LiAlO2 host without phase segregation. For the single-phase sample with 0.1 mol% doping of Cu, the lattice parameters a 5.17011(8) and c 6.2809(1) Å were calculated, which is a slight increase, compared to the lattice parameters of an undoped γ-LiAlO2 (5.1687 and 6.2679 Å for a and c, respectively). This change in lattice parameters is attributed to the low doping concentration and the small discrepancy of the ionic radii 0.76 and 0.77 Å for Li+ and Cu+ ions [39], respectively.
The radioluminescence spectra of the Cu-doped LiAlO2 samples, shown in Fig. 3a, feature a single broad emission band at around 360 nm, which corresponds to the interconfigurational transition of Cu+ ions from 3d9 4s1 to 3d10 [8, 38, 40]. This emission is observed even though the annealing atmosphere used for the preparation of these samples was air, suggesting that Cu+ ions can be stabilized by the LiAlO2 host lattice in the Li+ position. The highest radioluminescence intensity was observed for the sample with the lowest doping concertation tested, i.e., 0.1 mol%. The emission intensity of the samples with a higher doping concentration of 0.5 and 1.0 mol% was significantly lower, which is likely related to the presence of a secondary CuO phase in these samples, since radioluminescence is affected by defects in the scintillator host material. To stabilize a higher content of Cu+ ions in the LiAlO2 host and thus further improve the emission intensity, it could be beneficial to use a reducing annealing atmosphere during sample preparation.
A slightly different trend in emission intensities is observed within the Cu concentration series studied in the case of photoluminescence, shown in Fig. 3b. The excitation spectra show a broad excitation band at around 272 nm, corresponding to the Cu+ transition from 3d10 to 3d9 4s1. Emission spectra show the reverse transition (i.e. from 3d9 4s1 to 3d10) shifted towards lower energy. Contrary to radioluminescence, the intensity of photoluminescence increases up to the highest Cu-doping concentration. The photoluminescence decay curves are shown in Fig. 4. Their single exponential course and decay times within 16–20 μs match well the typical decay times of Cu+ ultraviolet luminescence in various hosts at room temperature (see the review by Bosi et al. in [41] and the references therein).
3.2 Cu:LiAl5O8
Figure 5 shows XRD patterns of Cu-doped LiAl5O8 ceramics. All reflections in the measured XRD patterns match well the low-temperature α-LiAl5O8 phase, according to PDF 04-014-9788. Within the tested doping concentration series, no phase segregation was observed and all samples were a single phase, containing only the α-LiAl5O8 phase with an inverse spinel structure and the P4332 space group. Similar to Cu:LiAlO2 samples, doping of Cu into the cubic α-LiAl5O8 host resulted in a slight increase in the lattice parameter a, as shown in Table 2.
The radioluminescence spectra of the copper-doped LiAl5O8 samples, shown in Fig. 6, feature an emission band at around 360 nm, which should correspond to the 4s13d9 → 3d10 transition of Cu+ ions, similarly to the luminescence of Cu:LiAlO2 samples. However, this emission band is not detected in photoluminescence measurements (Fig. 7) and also the photoluminescence decay curves (Fig. 8) do not produce the expected decay times of Cu+ ions. In addition, there are two overlapping bands in the radioluminescence spectra that peak at around 680 and 710 nm, which most likely correspond to the emission of Fe and Cr impurities, respectively. The intensity of the emission favors a lower doping concentration of copper in the LiAl5O8 host. However, the overall intensity of the emission is very weak for all Cu-doped samples compared to that of the BGO reference scintillator. The two samples that showed the highest emission intensity, i.e., samples with 0.05 and 0.075 mol% doping concentrations, were also synthesized using a reduction annealing atmosphere instead of air. However, the use of an H2 annealing atmosphere further deteriorated the already weak emission of Cu:LiAl5O8 samples (Fig. 6b). A slight change in the emission pattern was observed in the UV region for the 0.05 mol% doping when a reducing annealing atmosphere was used. This change might be related to an unidentified host or a defect emission of the polycrystalline material.
Photoluminescence excitation spectra (Fig. 7a), measured at the emission wavelength of 360 nm, showed an excitation band only for the 0.1 mol% Cu-doping concentration at 263 nm. The remaining doping concentrations did not feature any well-distinguished excitation bands at all. Moreover, photoluminescence emission spectra, measured at λexc. = 263 nm, featured mostly the 5d–5d emission of Fe3+ impurities with decay times within 4–8 ms, while the emission of copper ions was not observed or possibly present, but enveloped by other unknown emission bands in the case of 0.1 mol% doping. For this sample, the photoluminescence decay curves were measured (Fig. 8). Using both the microsecond xenon flashlamp and nanoLED excitation for slow (Fig. 8a) and fast (Fig. 8b) components, respectively, strongly non-exponential decays were measured, which were approximated by a sum of exponential functions. However, none of the decay times was close to the typical Cu+ values found above in Fig. 4 or reviewed in [41] and references therein, leaving the origin of this emission in question.
3.3 Cu:Li5AlO4
Among the three phases of lithium aluminate, the lithium-rich Li5AlO4 is by far the most challenging in terms of synthesis, which is also apparent from the low number of publications available. So far, the preparation of an undoped single-phase α- and β-Li5AlO4 was reported only by solid-state synthesis, while successful preparation of doped Li5AlO4 has not been published yet. In this work, we investigated preparation from a solution, since this approach offers superior homogeneity of the dopant distribution compared to that of a solid-state synthesis.
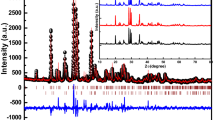
The Cu:Li5AlO4 preparation was first tested using the Pechini sol–gel route; however, when annealed at 800 °C in air, the samples melted. To investigate the cause, sample precursors prepared by the sol–gel method and annealed at 500 °C, were subjected to XRD and DTA/TG measurements. According to XRD, these precursors contain two well-distinguished phases after annealing at 500 °C, namely LiAl2(OH)7·xH2O and Li2CO3, the latter of which has a melting point of 723 °C, as confirmed by DTA (both measurements are shown in Fig. 9). Therefore, for our next experiments, we tested different heat treatments of the sol–gel prepared precursors below the melting point of Li2CO3, which should have produced the low-temperature α-Li5AlO4. However, the formation of lithium-rich Li5AlO4 was not observed even after prolonged annealing at 710 °C in air, as shown in Fig. 10. Instead, the samples contained a combination of Li2CO3, γ-LiAlO2, α-LiAlO2 and a trace amount of Li2CuO2. Using an inert nitrogen atmosphere during annealing, that is, a CO2-free atmosphere, prevented the formation of α-LiAlO2 and also decreased the amount of Li2CO3 phase in the sample. However, the formation of a lithium-rich Li5AlO4 was not achieved. As a result, the sol–gel method, although capable of synthesizing a wide variety of multicomponent oxide materials, was deemed unsuitable for the preparation of lithium-rich Li5AlO4.
XRD patterns of sol–gel prepared samples annealed at 710 °C in air and nitrogen atmosphere. The doping concentration of these samples was 0.1 mol% of Cu. Phases α-LiAlO2, γ-LiAlO2, Li2CO3, and Li2CuO2 were identified according to PDF 01-074-2232, PDF 04-009-6438, PDF 04-010-7186, and PDF 00-043-0266, respectively
The second approach tested the preparation of a metal nitrate solution followed by evaporation at 120 °C and calcination at 1000 °C in air. Using this technique, we were able to obtain samples containing the β-Li5AlO4 along with the secondary γ-LiAlO2 phase, as shown in Fig. 11. The loss of Li stoichiometry was probably caused by the volatility of Li2O, which led to a segregation of the Li-poor phase. Increasing the ratio of Li:Al in a starting solution above the stoichiometric value of 5:1 did not prevent the formation of the secondary γ-LiAlO2 phase. Furthermore, according to the Rietveld refinement, all samples contained a similar ratio of the β-Li5AlO4 and γ-LiAlO2 phases, despite the varying ratio of Li and Al in the starting solution. However, these samples, although not pure β-Li5AlO4, did exhibit significant hygroscopic properties. This behavior of lithium-rich Li5AlO4 is in line with previous studies reporting the air sensitivity of Li5AlO4 [42, 43]. Since the hygroscopic nature of this material would require the use of a controlled atmosphere, these samples were not measured for their optical properties and Li5AlO4 was not further investigated as a host material.
4 Conclusion
The copper-doped lithium aluminates γ-LiAlO2 and α-LiAl5O8 were prepared using the Pechini sol–gel method. For Cu:LiAlO2, only small doping concentrations resulted in a single-phase material; otherwise, a secondary CuO phase was formed. On the other hand, Cu:LiAl5O8 can be readily prepared with a doping concentration of up to 1 mol% Cu without phase segregation; however, its luminescence favors much lower doping concentrations. Overall, the luminescence properties of Cu:LiAlO2 are more pronounced with a distinct emission transition of Cu+ ions at 360 nm and decay times within 16–20 μs. In contrast, the Cu:LiAl5O8 samples featured mainly the emission of accidental impurities of Cr3+ and Fe3+. Single-phase Cu-doped Li5AlO4 samples could not be prepared due to the strong tendency of Li2O to evaporate. Not even the excess of lithium allowed the preparation of pure Cu-doped Li5AlO4 samples. Samples containing the β-Li5AlO4 and secondary γ-LiAlO2 phase exhibited significant hygroscopic properties, making Li5AlO4 an undesirable host material outside of a controlled atmosphere environment.
References
Mukhopadhyay S, Maurer R, Guss P, Kruschwitz C (2014) Review of current neutron detection systems for emergency response. In: Proc. SPIE 9213, Hard X-Ray, Gamma-Ray, and Neutron Detector Physics XVI. 92130T
Eijk CWE, Bessière A, Dorenbos P (2004) Inorganic thermal-neutron scintillators. Nucl Instrum Methods Phys Res Sect A: Accel Spectrom Detect Assoc Equip 529:260–267. https://doi.org/10.1016/j.nima.2004.04.163
Eijk CWE (2004) Inorganic scintillators for thermal neutron detection. Radiat Meas 59:337–342. https://doi.org/10.1016/j.radmeas.2004.02.004
Firk FWK, Slaughter GG, Ginther RJ (1961) An improved Li6-loaded glass scintillator for neutron detection. Nucl Instrum Methods 13:313–316. https://doi.org/10.1016/0029-554X(61)90221-X
Murray RB (1958) Use of Li6I(Eu) as a scintillation detector and spectrometer for fast neutrons. Nucl Instrum 2:237–248. https://doi.org/10.1016/0369-643X(58)90035-5
Syntfeld A, Moszynski M, Arlt R et al. (2005) 6LiI(Eu) in neutron and γ-ray spectrometry – a highly sensitive thermal neutron detector. IEEE Trans Nucl Sci 52:3151–3156. https://doi.org/10.1109/TNS.2005.860193
Yanagida T, Fujimoto Y, Koshimizu M et al. (2017) Comparative studies of optical and scintillation properties between LiGaO2 and LiAlO2 crystals. J Phys Soc Jpn 86:094201. https://doi.org/10.7566/JPSJ.86.094201
Fujimoto Y, Kamada K, Yanagida T et al. (2012) Lithium aluminate crystals as scintillator for thermal neutron detection. IEEE Trans Nucl Sci 59:2252–2255. https://doi.org/10.1109/TNS.2012.2198494
Knoll GF (2010) Radiation detection and measurement. John Wiley & Sons
Hirano S-I, Hayashi T, Kageyama T (1987) Synthesis of LiAlO2 powder by hydrolysis of metal alkoxides. J Am Ceram Soc 70:171–174. https://doi.org/10.1111/j.1151-2916.1987.tb04953.x
Jimenez-Becerril J, Bosch P, Bulbulian S (1991) Synthesis and characterization of γ-LiAlO2. J Nucl Mater 185:304–307
Kalyana Sundaram NT, Subramania A (2007) Nano-size LiAlO2 ceramic filler incorporated porous PVDF-co-HFP electrolyte for lithium-ion battery applications. Electrochim Acta 52:4987–4993. https://doi.org/10.1016/j.electacta.2007.01.066
Terada S, Nagashima I, Higaki K, Ito Y (1998) Stability of LiAlO2 as electrolyte matrix for molten carbonate fuel cells. J Power Sources 75:223–229. https://doi.org/10.1016/S0378-7753(98)00115-3
Dickens PT, Marcial J, McCloy J et al. (2017) Spectroscopic and neutron detection properties of rare earth and titanium doped LiAlO2 single crystals. J Lumin 190:242–248. https://doi.org/10.1016/j.jlumin.2017.05.047
Brik MG, Teng H, Lin H et al. (2010) Spectroscopic and crystal field studies of LiAlO2:Mn2+ single crystals. J Alloy Compd 506:4–9. https://doi.org/10.1016/j.jallcom.2010.06.160
Aoyama M, Amano Y, Inoue K et al. (2013) Synthesis and characterization of Mn-activated lithium aluminate red phosphors. J Lumin 136:411–414. https://doi.org/10.1016/j.jlumin.2012.12.012
Pejchal J, Fujimoto Y, Chani V et al. (2011) Crystal growth and luminescence properties of Ti-doped LiAlO2 for neutron scintillator. J Cryst Growth 318:828–832. https://doi.org/10.1016/j.jcrysgro.2010.11.053
Danek V, Tarniowy M, Suski L (2004) Kinetics of the α → γ phase transformation in LiAlO2 under various atmospheres within the 1073–1173 K temperatures range. J Mater Sci 39:2429–2435. https://doi.org/10.1023/B:JMSC.0000020006.46296.04
Kulkarni NS, Besmann TM, Spear KE (2008) Thermodynamic optimization of lithia-alumina. J Am Ceram Soc 91:4074–4083. https://doi.org/10.1111/j.1551-2916.2008.02753.x
Singh V, Sivaramaiah G, Rao JL et al. (2017) Optical and EPR spectroscopic studies of deep red light emitting Fe-doped LiAl5O8 phosphor prepared via propellant combustion route. J Elec Mater 46:1525–1531. https://doi.org/10.1007/s11664-016-5192-z
Li X, Jiang G, Zhou S et al. (2014) Luminescent properties of chromium(III)-doped lithium aluminate for temperature sensing. Sens Actuators B: Chem 202:1065–1069. https://doi.org/10.1016/j.snb.2014.06.053
Kniec K, Tikhomirov M, Pozniak B et al. (2020) LiAl5O8:Fe3+ and LiAl5O8:Fe3+, Nd3+ as a new luminescent nanothermometer operating in 1st biological optical window. Nanomaterials (Basel) 10:189. https://doi.org/10.3390/nano10020189
Teixeira VC, Andrade AB, Ferreira NS et al. (2019) X-ray excited optical luminescence and morphological studies of Eu-doped LiAl5O8. Phys B: Condens Matter 559:62–65. https://doi.org/10.1016/j.physb.2019.01.050
Mu Z, Hu Y, Chen L et al. (2012) Luminescence and energy transfer in phosphor LiAl5O8: Ce3+, Dy3+. Radiat Meas 47:426–429. https://doi.org/10.1016/j.radmeas.2012.03.027
Silva AJS, Nascimento PAM, Andrade AB et al. (2018) X-ray excited optical luminescence changes induced by excess/deficiency lithium ions in rare earth doped LiAl5O8. J Lumin 199:298–301. https://doi.org/10.1016/j.jlumin.2018.03.066
Singh V, Pathak MS, Singh N et al. (2018) Effect of annealing on photoluminescence properties of combustion synthesized ultraviolet-emitting cerium-ion-doped LiAl5O8 phosphor. Optik 152:9–15. https://doi.org/10.1016/j.ijleo.2017.08.108
Braun PB (1952) A superstructure in spinels. Nature 170:1123–1123. https://doi.org/10.1038/1701123a0
Datta RK, Roy R (1963) Phase transitions in LiAl5O8. J Am Ceram Soc 46:388–390. https://doi.org/10.1111/j.1151-2916.1963.tb11757.x
Famery R, Queyroux F, Gilles J-C, Herpin P (1979) Etude structurale de la forme ordonnée de LiAl5O8. J Solid State Chem 30:257–263. https://doi.org/10.1016/0022-4596(79)90107-5
Pott GT, McNicol BD (1972) Zero‐phonon transition and fine structure in the phosphorescence of Fe3+ ions in ordered and disordered LiAl5O8. J Chem Phys 56:5246–5254. https://doi.org/10.1063/1.1677027
Ávalos-Rendón T, Casa-Madrid J, Pfeiffer H (2009) Thermochemical capture of carbon dioxide on lithium aluminates (LiAlO2 and Li5AlO4): a new option for the CO2 absorption. J Phys Chem A 113:6919–6923. https://doi.org/10.1021/jp902501v
Wang YJ, Liu JP, Kan MX, Liu ZQ (2013) The synthesis of lithium aluminate materials and its performance of CO2 absorption. Adv Mater Res 669:115–118
Sharma A, Chourasia NK, Sugathan A et al. (2018) Solution processed Li5AlO4 dielectric for low voltage transistor fabrication and its application in metal oxide/quantum dot heterojunction phototransistors. J Mater Chem C 6:790–798. https://doi.org/10.1039/C7TC05074G
Andreev OL, Zelyutin GV, Martem’yanova ZS, Batalov NN (2001) Electrical conductivity of Li6BeO4–Li5AlO4 solid solutions. Inorg Mater 37:177–179. https://doi.org/10.1023/A:1004122013795
La Ginestra A, Lo Jacono M, Porta P (1972) The preparation, characterization, and thermal behaviour of some lithium aluminum oxides: Li3AlO3 and Li5AlO4. J Therm Anal 4:5–17. https://doi.org/10.1007/BF02100945
Ogawa S, Shin-mura K, Otani Y, et al. (2017) Synthesis of High Purity Li5AlO4 Powder by Solid State Reaction Under the H2 Firing. In: Lin H-T, Matyáš J, Katoh Y, Vomiero A (eds). Ceramic Materials for Energy Applications VI. John Wiley & Sons, Ltd, pp. 49–60. https://doi.org/10.1002/9781119321774.ch6
Follstaedt DM, Biefeld RM (1978) Nuclear-magnetic-resonance study of Li+ motion in lithium aluminates and LiOH. Phys Rev B 18:5928–5937. https://doi.org/10.1103/PhysRevB.18.5928
Fujimoto Y, Yanagida T, Kamada K, Pejchal J (2013) Radiation response of transition metals-doped lithium aluminate crystals. In: 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC). IEEE, Seoul, Korea (South), pp. 1–3. https://doi.org/10.1109/NSSMIC.2013.6829660
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767. https://doi.org/10.1107/S0567739476001551
Gomez S, Urra I, Valiente R, Rodriguez F (2010) Spectroscopic study of Cu2+ and Cu+ ions in high-transmission glass. Electronic structure and Cu2+ /Cu+ concentrations. J Phys: Condens Matter 22:295505. https://doi.org/10.1088/0953-8984/22/29/295505
Bosi L, Bosi FL, Gallo D, Zelada M (2001) Review of emission and lifetime data concerning Cu+ fluorescence in alkali halides. Phys Status Solidi (B) 223:821–829. https://doi.org/10.1002/1521-3951(200102)223:3<821::AID-PSSB821>3.0.CO;2-W
Johnson RT, Biefeld RM (1979) Ionic conductivity of Li5AlO4 and Li5GaO4 in moist air environments: potential humidity sensors. Mater Res Bull 14:537–542. https://doi.org/10.1016/0025-5408(79)90197-1
Wang X, Wang J, Li F et al. (2020) Influence of cold sintering process on the structure and properties of garnet-type solid electrolytes. Ceram Int 46:18544–18550. https://doi.org/10.1016/j.ceramint.2020.04.160
Persson K (2014) Materials Data on Li5AlO4 by Materials Project. The Materials Project, United States. https://doi.org/10.17188/1289042
Persson K (2014) Materials Data on LiAlO2 by Materials Project. The Materials Project, United States. https://doi.org/10.17188/1206753
Persson K (2014) Materials Data on LiAl5O8 by Materials Project. The Materials Project, United States. https://doi.org/10.17188/1263228
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Cryst 44:1272–1276. https://doi.org/10.1107/S0021889811038970
Acknowledgements
This work was financially supported by the Ministry of the Interior of the Czech Republic (Grant No. VI20192022152). Partial support from the European Structural and Investment Funds Project No. CZ.02.1.01/0.0/0.0/16_026/0008390 and from Specific University Research Grant No. A2_FCHT_2021_003, both by the Ministry of Education, Youth and Sports, is also gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study and to the preparation of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thoř, T., Rubešová, K., Jakeš, V. et al. Preparation and luminescence characterization of Cu-doped lithium aluminate ceramics within the Li2O-Al2O3 system. J Sol-Gel Sci Technol 103, 898–907 (2022). https://doi.org/10.1007/s10971-022-05905-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05905-x