Abstract
Hydrofluoroethers (HFEs) represent a new family of promising engineering fluids suitable for technical cleaning and cooling of electronic and magnetic devices or as admixtures in refrigerant blends. Here, we report accurate data for the liquid density at 0.1 MPa and temperatures from \(273.15\,\text{K}\) to \(343.15\,\text{K}\) for a series of five HFEs, namely, HFE-7000, HFE-7100, HFE-7200, HFE-7300, and HFE-7500. A highly sensitive vibrating tube densimeter with a borosilicate glass U-tube calibrated according to the procedure by Prokopová et al. (J Chem Thermodyn 173:106855, 2022) provided density data with an expanded uncertainty (\(k=2\)) of \(0.13\,\text{kg}\cdot \text{m}^{-3}\). Influences such as sample degassing, water content, or sample temperature before its dosing into the densimeter are discussed. Thanks to the high sensitivity of the used densimeter, an unexpected shift in the density of different HFE-7100 and HFE-7200 liquid samples was detected. Unlike other HFEs, HFE-7100 and HFE-7200 are mixtures of two hardly separable isomers, which were so far considered having identical thermophysical properties. Utilizing nuclear magnetic resonance spectroscopy, the ratio of n-isomer and iso-isomer was inspected for various liquid samples. In the range of iso-isomer mole fraction from 0.61 to 0.77, the new measurements revealed density differences of more than \(5\,\text{kg}\cdot \text{m}^{-3}\) in case of HFE-7100 and of about \(3\,\text{kg}\cdot \text{m}^{-3}\) in case of HFE-7200. Consequently, for some applications, the properties of different HFE isomers cannot be considered identical. The Rackett-type correlation for the saturated liquid density was fitted using the new and the literature data.
Similar content being viewed by others
1 Introduction
The fluorinated ethers, also known under the commercial name \(\text{Novec}^{TM}\) introduced by the 3M company [1], are receiving increasing attention from researchers and engineers as promising alternatives to chloro-fluoro-hydrocarbons over the past two decades. Hydrofluoroethers (HFEs) are excellent heat transfer fluids suitable for cooling of electronic and magnetic devices, cleaning and blowing agents, or low-boiling components in refrigerant blends. In comparison with other commonly used engineering fluids, HFEs cause zero ozone depletion and have relatively low global warming potential (GWP) and high dielectric constant. However, it should be noted that since HFEs belong to per- and polyfluoroalkyl substances (PFASs), their wider use may be limited in future. For an effective and reliable use of fluorinated ethers, the accurate description of their thermodynamic properties such as density, vapor pressure, excess molar volume, and transport properties such as viscosity or surface tension is crucial. Several research groups have recently provided valuable experimental data on some of these properties [2,3,4,5] and developed predictive models mostly based on the SAFT-type equations of state [6,7,8]. Nevertheless, the introduced models have limited ranges of validity and for some applications unsatisfactory accuracy. The available property data are still rather limited in order to develop and verify accurate models valid over wide temperature and pressure ranges such as empirical multiparameter equations of state [9] as included in NIST’s REFPROP package [10].
An interesting phenomenon that has not been addressed in detail is the isomeric composition of some HFE fluids, namely, HFE-7100 which is a trade name of binary isomer mixture of 1,1,1,2,2,3,3,4,4-nonafluoro-4-methoxybutane and 1,1,1,2,3,3-hexafluoro-3-methoxy-2-(trifluoromethyl)propane, and HFE-7200 being a trade name of binary isomer mixture of 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane and 1-ethoxy-1,1,2,3,3,3-hexafluoro-2-(trifluoromethyl)-propane. Since the isomers of the HFE-7100 and HFE-7200 mixtures are similar substances with the same functional groups, the relative volatility of the substances can be expected to be close to 1. As a result, the isomers are difficult to separate from each other, e.g., by gas-liquid chromatography [11]. Therefore, most producers, including the 3M company, declare HFE-7100 and HFE-7200 as mixtures of two inseparable isomers with essentially identical properties. Consequently, the thermophysical properties of these fluids such as the boiling point or liquid density are reported as for a pure substance [12, 13]. On the other hand, our new experiments slightly undermine this simplification. Sensitive measurements carried out with the vibrating tube densimeter (VTD) revealed density differences of up to \(6.4\,{\text{kg}\cdot \text{m}^{-3}}\) and \(3.1\,{\text{kg}\cdot \text{m}^{-3}}\) between different batches of HFE-7100 and HFE-7200, respectively. These differences are remarkably larger compared to other single-isomer HFE fluids, i.e., HFE-7000, HFE-7300, and HFE-7500, which showed only small differences in densities of up to \(0.6\,{\text{kg}\cdot \text{m}^{-3}}\) between the different production batches. The detected five to ten times higher discrepancies in the liquid density point to the effect of isomeric composition of HFE-7100 and HFE-7200.
The main goal of this work is to provide reference liquid density data at 0.1 MPa for a series of HFE fluids, specified in Table 1, and to shed light on the influence of HFE isomeric composition. The composition of the mixture may change during sample handling due to different evaporation of individual components. We have therefore tried to trace various experimental effects such as temperature during the sample handling or different level of degassing that can influence the sample composition and the measured liquid density. Accurate data for liquid density are necessary for the next step of description of HFE properties, e.g., for the development of multiparameter equations of state [9].
2 Experimental
A highly sensitive vibrating tube densimeter with a borosilicate glass U-tube was used for the measurement of liquid density at barometric pressure for a series of different HFE samples. The new data were collected in the temperature range from 273 K to the vicinity of the normal boiling point.
2.1 Materials
Description of all samples of five different HFE fluids and one pure isomer 1,1,2,3,3,3-hexafluoro-1-methoxy-2-(trifluoromethyl)propane, here called HFE-7100-iso, including their purity and water content is summarized in Tables 1 and 2. No further purification was applied except for the degassing of 5 ml sample in a 10-ml plastic syringe by applying repeatedly a slight vacuum for about 10 s. Samples used for the reference measurement of liquid density at 0.1 MPa were degassed four times. Further details on the sample degassing and the change of sample composition in case of two-isomer liquids due to evaporation are given in the Results section. The water content was measured utilizing the Karl-Fischer coulometric titrator (Mettler Toledo C30) or taken from analytical certificates. It is recommended to have at least 50 μg of water in the sample in case of coulometric analysis. For very dry samples, for which the measured water content was under the detection limit (UDL) of the titrator, a water mass fraction lower than \(10\times 10^{-6}\) was assumed. With regard to values given in Table 2, the HFE samples can be considered almost water-free. All samples were stored in the refrigerator at a temperature of 278 K. The samples were taken directly from the bottle with a sterile syringe, quickly degassed and dispensed into the VTD U-tube or further processed in order to intentionally alter the composition of isomeric mixtures.
The molar ratio of structural isomers in HFE-7100 and HFE-7200 samples were determined using the NMR analysis. 19F NMR spectra (75.72 MHz, CDCl3) were measured using a 80 MHz instrument Spinsolve 80 ULTRA (Magritek, Germany) at a room temperature. The chemical shifts (\(\delta \)) are given in ppm. As depicted in Figs. 1 and 2, signals at − 80.2 ppm (iso-) and − 86.6 ppm (n-) were used to determine the isomer ratio of HFE-7100. In case of HFE-7200 isomers, signals at − 77.3 ppm (iso-) and − 83.8 ppm (n-) were employed [14].
2.2 Vibrating Tube Densimeter
Vibrating tube densimeters are very sensitive instruments for the measurement of fluid densities ranging from gases to dense liquids. When carefully calibrated, the standard combined uncertainties in the order of \(\pm \, 0.10\,\text{kg}\cdot \text{m}^{-3}\) can be achieved [15,16,17] for the high-pressure instruments equipped with the metal U- or V-shaped vibrating tubes. The low-pressure units with glass tubes can attain even one order of magnitude lower standard combined uncertainties of \(\pm \, 0.010\,\text{kg}\cdot \text{m}^{-3}\) [18,19,20].
In this work, the vibrating tube densimeter of Anton Paar, model DMA 5000 M [21] with a borosilicate glass U-tube was used. With a resolution down to \(0.001\,\text{kg}\cdot \text{m}^{-3}\), the instrument belongs to the most sensitive VTDs. The densimeter was operated and calibrated according to the procedure described in previous work [20]. The calibration technique inspired by the approach of Fritz et al. [22] employs a series of repeated measurements with ultra-pure water and dry air. In short, the fluid density \(\rho \) can be determined from the following equation
where \(PQ = \tau /\tau _{\text{ref}}\) is a relative period of oscillation, A and B are temperature-dependent parameters obtained from the reference measurements with water and air over the temperature range from 273 K to 363 K, and \(\Delta D_0(T) = D_{0,\text{sample}}(T)-D_{0,\text{air}}(T)\) is the damping difference of the measured sample and dry air at a given temperature T. \(V_1\) and \(V_2\) are the damping coefficients reflecting the influence of sample viscosity.
The HFE samples were measured in the range from \(T=273\) K to the temperatures 4 K to 60 K below the normal boiling point depending on the sample volatility. Due to the rather high evaporation of most HFE samples, both the inlet and outlet openings of the VTD U-tube were loosely closed with teflon plugs to guarantee constant sample composition during the whole measurement. Several experimental setups leading to the prevention of evaporation of the sample from the U-tube were tested. The differences in sample densities obtained in the way of the U-tube plugged and unplugged were below \(0.030\,{\text{kg}\cdot \text{m}^{-3}}\), i.e., they lie within the expanded uncertainty (\(k=2\)) of the density data of \(0.13\,{\text{kg}\cdot \text{m}^{-3}}\). Each sample measurement was followed by water measurements and at least every 10 days by dry air measurements to check the calibration parameters and stability of VTD over time. The final densities of the HFE samples were calculated based on the temperature dependent parameters A and B obtained from the measurement campaign of at least 5 water and 3 air measurements.
3 Results and Discussion
Tables 3, 4, 5, 6, and 7 summarize the experimental data for the liquid density of five HFE fluids. The density data represent the average values of two to three measurements with different samples from each batch specified in Table 2. Since the measurements were carried out with the U-tube inputs loosely plugged, the liquid pressure can be considered slightly higher than the reported barometric pressure measured with a high-precision external pressure gauge Druck DPI 142 (GE, USA). An internal Pt100 temperature probe located close to the tip of the U-tube was inspected as described in previous work [20].
3.1 Isomeric Composition of HFE-7100 and HFE-7200
Tables 8 and 9 provide the density data of different HFE-7100 and HFE-7200 samples depending on the mole fraction of the iso-isomer at three temperatures of 283 K, 298 K, and 303 K. New data are compared with the literature sources containing the information on the composition of the isomeric mixture. The composition dependence of the liquid density at 0.1 MPa of all known samples is depicted in Fig. 3. As can be seen, the density of both liquids gradually increases with the higher content of the iso-isomer. In the typical composition range with the mole fraction of iso-isomer from 0.6 to 0.8, the detected density difference reaches \(5\,\text{kg}\cdot \text{m}^{-3}\) and \(3\,\text{kg}\cdot \text{m}^{-3}\) in case of HFE-7100 and HFE-7200, respectively. These differences considerably overreach the expanded uncertainty of most experimental data, which are typically in the range of 0.1 to \(1\,\text{kg}\cdot \text{m}^{-3}\). It should be noted that the influence of isomeric composition of HFE-7100 and HFE-7200 can be neglected in most engineering applications. On the other hand, it needs to be considered in the development of accurate property models such as empirical multiparameter equations of state [9]. Moreover, due to favorable properties such as low hygroscopicity, HFE fluids are viewed as good candidates for calibration liquids, e.g., for accurate density measurement. If so, we recommend focusing predominantly on the single isomeric HFE fluids.
3.2 Temperature Correlation for Saturated Liquid Density of HFEs
The new density data obtained at 0.1 MPa together with the low-pressure data taken from the literature were fitted with the Rackett-type correlation [26]. The correlation, given in the form of Eq. 2, provides good predictions for the saturated liquid density of all five HFEs.
In Eq. 2, parameter A approximates the critical density \(\rho _{\text{crit}}\) and B can be considered as the critical compressibility factor \(Z_{\text{crit}}\). We note that Vetere [26] employed a universal value of exponent N of 2/7 that can be used for less described liquids. In general, various values for N usually in the range from 0.1 to 0.5 are used when representing low-pressure experimental data, e.g., as reported by Outcalt et al. [4, 27]. Except for HFE-7000, exponent N was set equal to 0.30 which provides good representation of all available density data by achieving acceptable reproduction of the critical point estimated by Aminian et al. [28]. Table 10 summarizes the parameters of Eq. 2 for the five selected HFEs obtained from the minimization of the following objective function
with \(N_{\text{exp}}\) standing for the number of density data points, \(U_i\) for the expanded uncertainty \((k=2)\), and \(w_i\) for the weight lying between 0 and 1. The weight equaled to 1.0, except for the data points from the previous work [6] whose weight was set to 0.1—more details are given in the discussion of Figs. 4, 5, 6, 7, and 8. Average values of the standard deviation of the correlated parameters are also provided. Critical temperature \(T_{\text{crit}}\) was taken from Aminian et al. [28]. Values of parameter A are approximately 4 % higher than the critical density \(\rho _{\text{crit}}\) estimated recently by Aminian et al. [28]. It shall be emphasized that correlation (2) is based on the experimental data in the temperature range typically from 273 K to 363 K. However, it is expected to provide reasonable prediction for the saturated liquid density also at higher temperatures as it follows the temperature trend toward the critical point density.
Table 10 also provides the values for the expanded relative deviation of Eq. 2 from the correlated experimental data. The relative deviations for HFE-7100 and HFE-7200 are considerably larger compared to other three single isomeric HFEs. This indicates greater scatter of the density data due to the two-isomer composition of HFE-7100 and HFE-7200.
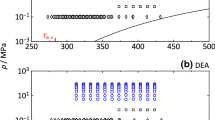
Figures 4, 5, 6, 7, and 8 compare the literature data and the new data for the liquid density at 0.1 MPa and the saturated liquid density of five selected HFEs with the Rackett-type correlation (2). The horizontal black dashed lines indicate the expanded relative deviation of the correlation given in Table 10. The blue dash-dotted lines correspond to correlations given as linear functions of temperature in 3M datasheets [12, 13, 29,30,31]. As can be seen, the 3M correlations deviate from the available experimental data by several tenths of a percent. The largest discrepancies can be seen in case of HFE-7100 as shown in Fig. 5. It should be noted that the 3M correlations were developed before most of the experimental data were published and as such should be viewed as preliminary engineering correlations. Out of recent experimental data, the measurements by Rausch et at. [2], carried out with a VTD under the saturated conditions, were found to show the best internal consistency over wide temperature range from 273 K to 363 K for all five selected HFEs. We note that our previous data collected with the single-sinker buoyancy method [6] show slightly different temperature slopes than other data for most HFEs. We suspect that this discrepancy occurred due to possible temperature gradients and convection in the relatively large liquid sample with volume of around 100 ml. On the other hand, the buoyancy method is not influenced by the sample viscosity, which may cause substantial errors in the data obtained with a VTD [19]. The good agreement between the buoyancy method and the new VTD data in the vicinity of the laboratory temperature of 298 K, where the possible temperature gradients were the lowest, provides additional verification of the viscosity correction employed in Eq. 1 for relatively low-viscosity HFE fluids. Furthermore, the agreement of both measuring techniques close to 298 K and with the data by Rausch et al. [2] for most of the single-isomer HFE samples confirms the VTD calibration according to Prokopová et al. [20], which was extrapolated to rather high densities from 1300 to \(1700\,\text{kg}\cdot \text{m}^{-3}\).
Relative deviation of the Rackett-type correlation (2) for HFE-7000 from the densities taken from literature [2, 3, 6, 32, 33] new measurements, correlation by 3M manufacturer [29] and the preliminary multiparameter equation of state [10, 34]. Black dashed lines indicate the expanded relative deviation of Eq. 2
A preliminary, i.e., so far not officially published, multiparameter equation of state by Zhou and Lemmon [34] is available in REFPROP package v.10 [10] for HFE-7000. The predictions of the equation of state for the saturated liquid density are represented by the orange solid line in Fig. 4 and show quite good agreement with the new measurements in the temperature range from 273 to 303 K although our new data were not used to fit this equation of state. At higher temperatures, the equation shows an increasing deviation from the Rackett-type correlation (2), which follows the trend of the data by Rausch et at. [2].
Relative deviation of the Rackett-type correlation (2) for HFE-7100 from the densities taken from literature [2, 5, 6, 23, 24, 32, 35, 36], new measurements, correlation by 3M manufacturer [12], and the multiparameter equation of state by Huber and Lemmon [37]. Black dashed lines indicate the expanded relative deviation of Eq. 2
Another preliminary multiparameter equation of state has been recently introduced for HFE-7100 (denoted also as RE449mccc) in the supplement of a publication by Huber and Lemmon [37]. Unlike in case of HFE-7000, the equation of state follows the trend of the density data by Rausch et al. [2] over the entire temperature range as shown in Fig. 5. Similarly as shown in Fig. 3a, the influence of the isomeric composition of HFE-7100 on the experimental data can be clearly seen. The liquid density systematically increases with the increasing mole fraction of iso-isomer in the HFE-7100 samples. The data with similar iso-isomer mole fraction of around 60 % to 62 % by Cendon et al. [24], Pineiro et al. [23], and Rausch et al. [2] agree well with each other. The new data with the mole fractions from 67 % to 100 % are shifted to higher densities. For some of the data sources, the isomeric composition could not be traced. However, from the trend of the data shown in Fig. 5, one can assume that the iso-isomer mole fraction could be around 60 %, 69 %, and 76 % in case of the data by Qi et al. [32], Tanaka [35], and Vinš et al. [6], respectively. Unfortunately, in case of the first batch (no. 25584) measured in this work, the isomeric composition was not investigated either. One can assume that the iso-isomer mole fraction was approximately 75 %. Based on private communications, the samples measured by Muñoz-Rujas et al. [5] should have the iso-isomer mole fraction of around 52 %. However, the data show remarkably good agreement with other data with the iso-isomer mole fraction around 60 %. The possible composition of the samples employed by Shiflet and Yokozeki [36] is hard to judge due to the larger scatter of data.
A similar dependence of liquid density on isomeric composition can be seen in Fig. 6 for the other two-isomer component HFE-7200. A gradual increase in density can be seen with the increasing mole fraction of the iso-isomer. The data by Rausch et al. [2] and Muñoz-Rujaz et al. [25] with the iso-isomer mole fraction between 61 % and 62 % are in good agreement. The new data obtained in this work for the samples with an iso-isomer mole fraction between 67 % and 73 % are systematically higher by approximately 0.1 to 0.2 %. Based on the data shown in Fig. 6, one can again guess the isomeric composition of other data sources. The data by Fang et al. [38] and Pineiro et al. [23] seem to have an iso-isomer mole fraction of around 61 % due to the remarkably good agreement with the data by Rausch et al. [2] and Muñoz-Rujaz et al. [25]. Our previous data obtained with the single-sinker buoyancy method [6] seem to have the mole fraction of around 72 %.
Figure 7 shows the density data for HFE-7300 compared to the Rackett-type correlation (2). As can be seen, all experiments are in very good agreement except for the different temperature slope in case of the single-sinker buoyancy data from the previous study [6], which was discussed above. The two batches of HFE-7300 specified in Table 2 show a slight mutual offset of about \(0.5\,\text{kg}\cdot \text{m}^{-3}\). As the batches have comparable purity, low water content and were handled in the same manner, the difference is assumed to be the batch dependency similar to other studies, e.g., by Sommer et al. [40] for toluene. In case of HFE-7300, the 3M correlation [30] provides relatively good predictions over the temperature range from 275 K to 345 K.
The density data for HFE-7500 compared to correlation (2) are provided in Fig. 8. The new data for batch no. 21099 are in excellent agreement with the data by Rausch et al. [2] and Muñoz-Rujaz et al. [41] over the whole temperature range. The densities of the other two batches are slightly shifted by about 0.4 to \(0.5\,\text{kg}\cdot \text{m}^{-3}\) which is considered to be a batch dependency as observed for HFE-7300. The data by Lafitte et al. [3] and Fang et al. [38] show only slight discrepancies which are, except for a single point by Fang et al. at 293 K, fully within the interval of the expanded relative deviation of the Rackett-type correlation (black dashed lines in Fig. 8). The single-sinker buoyancy data [6] and the data by Shiflett and Yokozeki [36] show larger deviations. Both datasets [6, 36] have rather high expanded uncertainties of around 0.5 to \(4.0\,\text{kg}\cdot \text{m}^{-3}\). Their relevance was therefore lowered in the development of the density correlation (2). Besides, the weight \(w_i\) of the single-sinker buoyancy data [6] was lowered to 0.1, as discussed above.
3.3 Other Effects Influencing Measurements with a VTD at 0.1 MPa
Due to the high sensitivity of the VTD used, additional factors affecting the density measurement could be examined. The high resolution of \(0.001\,\text{kg}\cdot \text{m}^{-3}\) enables to inspect, for example, the gradual influence of sample degassing or the evaporation of two-isomeric samples at elevated temperatures.
3.3.1 Sample Degassing
As mentioned in section 2.1, the 5-ml liquid samples were degassed by applying repeatedly a slight vacuum in a 10-ml syringe. Our aim was to inspect the degree of degassing depending on the number of vacuum cycles and its influence on the measured liquid density. The samples, tempered inside a refrigerator at a constant temperature of 278 K, were taken from the 1-liter bottle, quickly degassed by different numbers of vacuum cycles in a syringe and applied directly into the VTD U-tube. The liquid density was measured at three different temperatures from 293 to 303 K. Figure 9 shows the observed variation of density depending on the number of degassing cycles for the two-isomer liquid HFE-7100 and the single-isomer liquids HFE-7000, HFE-7300, and iso-isomer of HFE-7100-iso with regard to the non-degassed samples.
Independent of the isomeric composition, almost identical density increase was observed for all samples taken from the bottle tempered at 278 K. A maximum increase of density of \(0.75\,{\text{kg}\cdot \text{m}^{-3}}\) was observed after 10 degassing cycles, with the steepest change occurring after the first 4 cycles. The measured density did not change any further with increasing number of cycles for any of the samples including HFE-7100. This indicates that the degassing technique did not affect the isomeric composition of the two-isomer sample and the increase in density corresponds only to sample degassing. To verify this, an additional experiment with two samples of single-isomer liquids HFE-7000 and HFE-7100-iso tempered at the laboratory temperature of 297 K was performed. In this case, the degassing technique shows a similar trend, however, with a lower increase in density of around \(0.5\,\,{\text{kg}\cdot \text{m}^{-3}}\). This is considered to be due to the lower solubility of air in HFEs at higher temperature.
These experimental tests showed that regardless of the possible isomeric composition or the temperature at which the sample was degassed, the liquid density increased approximately by 0.40 \(\,{\text{kg}\cdot \text{m}^{-3}}\) and 0.65 \(\,{\text{kg}\cdot \text{m}^{-3}}\) after 4 degassing cycles (i.e., standard degassing approach described in Sect. 2.1) at the degassing temperature of 278 K and 298 K, respectively. We note that the additional change in density due to further degassing cycles lies in the expanded uncertainty of the data presented.
3.3.2 Sample Temperature Before Its Dosing into the VTD U-tube
Another experimental test focused on the temperature of the sample and its handling prior to dosing into the VTD U-tube. Application of the liquid sample from a bottle into the densimeter using a syringe typically takes about 1 to 3 min, including degassing, flushing the U-tube with fresh sample, and capping the U-tube in- and outlet. Two-isomer mixture HFE-7100 (b.n. 24865) and single-component HFE-7300 (b.n. 20176) were measured at two different initial temperatures: once stored at the laboratory temperature of 298 K and once refrigerated at 278 K. The samples were not degassed in this case in order to speed up the sample handling. The density measurements at each storage temperature were repeated three times using the same procedure over the temperature range from 273 to 323 K. The density of the refrigerated samples was approximately \(0.025\,{\text{kg}\cdot \text{m}^{-3}}\) lower compared to the warmer samples. This tiny difference, lying well within the expanded uncertainty of the measured density, is believed to be due to the higher amount of air dissolved in the colder samples. It follows that the quick sample handling and the initial storage temperature do not affect the accuracy of the obtained density data.
The effect of exposure time to ambient air during the sample handling was inspected using the samples heated for 30 and 60 minutes in an uncovered beaker at a temperature of 308 K prior to dosing into the densimeter. For both HFE liquids, the density measured after 60 minutes was slightly higher compared to that after 30 minutes. The difference, which did not exceed a value of \(0.040\,{\text{kg}\cdot \text{m}^{-3}}\), appears to be due to the longer release of air dissolved in the liquid samples to the environment. On the other hand, a considerable difference was observed between HFE-7100 and HFE-7300 when comparing the quickly handled samples described in the previous paragraph with samples heated to \(T=308\) K for tens of minutes. In the case of single-isomer HFE-7300, the density of sample heated to \(T=308\) K was only \(0.057\,{\text{kg}\cdot \text{m}^{-3}}\) higher than in case of quickly dosed samples with the initial temperature of 298 K or 278 K. In contrast, the density of two-isomer HFE-7100 showed a noticeable increase of \(0.275\,{\text{kg}\cdot \text{m}^{-3}}\) due to the heating of the sample in ambient air. Although it is difficult to confirm, it appears that this five times higher difference was caused by the change of composition during evaporation of the HFE-7100 samples rather than by a reduced content of dissolved air.
In summary, exposure of the sample to the ambient air must be kept as low as possible, especially when accurate and reproducible density measurements of liquid mixtures are to be performed using the sensitive barometric VTDs.
4 Conclusion
New data for the liquid density for five different hydrofluorethers were measured using a vibrating tube densimeter Anton Paar, model DMA 5000 M. The density data were measured at approximately 0.1 MPa over the temperature range from 273 K to the vicinity of the normal boiling point with an expanded combined uncertainty (\(k=2\)) of \(0.13\,\text{kg}\cdot \text{m}^{-3}\). Due to the high sensitivity of the employed VTD and its careful calibration according to the procedure introduced in previous work [20], several interesting effects influencing the measured density were investigated on different batches of HFE samples. HFE-7100 and HFE-7200 are binary mixtures of two hardly separable isomers, whose thermophysical properties have been considered identical so far. However, new experiments showed a systematic shift in the liquid density of HFE-7100 and HFE-7200 depending on the mole fraction of the iso-isomer. In the typical range of iso-isomer mole fractions between 60 % and 100 %, the density varies by more than \(5\,\text{kg}\cdot \text{m}^{-3}\) and \(3\,\text{kg}\cdot \text{m}^{-3}\) in case of HFE-7100 and HFE-7200, respectively. Other experimental tests indicated the influence of sample handling before dosing into the VTD U-tube such as degassing, storage temperature, and exposure time to ambient air.
Based on the literature data for the low-pressure and the saturated liquid density and the new measurements, the Rackett-type correlation (2) was developed. The correlation provides good estimates for the saturated liquid density of all five HFEs. The expanded (\(k=2\)) relative deviation from the experimental data is lower than 0.2 % for all HFEs, except for HFE-7100 where the isomeric composition causes larger deviation. The new experimental data can be used in possible improvement of the preliminary multiparameter equations of state for HFE-7000 [34] and HFE-7100 [37] implemented in REFPROP [10], when especially the influence of the isomeric composition of HFE-7100 should be taken into account.
References
3M Science, 3M Novec Engineered Fluids—Performance, Sustainability, Safety. J344361. Electronics Materials Solutions Division, Bracknell (2017)
M.H. Rausch, L. Kretschmer, S. Will, A. Leipertz, A.P. Fröba, Density, surface tension, and kinematic viscosity of hydrofluoroethers HFE-7000, HFE-7100, HFE-7200, HFE-7300, and HFE-7500. J. Chem. Eng. Data 60, 3759–3765 (2015)
T. Lafitte, F. Plantier, M.M. Piñeiro, J.-L. Daridon, D. Bessières, Accurate global thermophysical characterization of hydrofluoroethers through a statistical associating fluid theory variable range approach, based on new experimental high-pressure volumetric and acoustic data. Ind. Eng. Chem. Res. 46, 6998–7007 (2007)
S.L. Outcalt, Compressed-liquid density measurements of 3-ethoxy-1,1,1,2,3,4,4,5,5,6,6,6-dodecafluoro-2-trifluoromethylhexane. J. Chem. Eng. Data 59, 2087–2094 (2014)
N. Muñoz-Rujas, F. Aguilar, J.-P. Bazile, E.A. Montero, Liquid density of mixtures methyl nonafluorobutyl ether (HFE-7100)+ 2-propanol at pressures up to 140 MPa and temperatures from 298.15 K to 393.15 K. Fluid Phase Equilib. 429, 281–292 (2016)
V. Vinš, A. Aminian, D. Celný, M. Součková, J. Klomfar, M. Čenský, O. Prokopová, Surface tension and density of dielectric heat transfer fluids of HFE type-experimental data at 0.1 MPa and modeling with PC-SAFT equation of state and density gradient theory. Int. J. Refrig. 131, 956–969 (2021)
D. Jovell, R. Gonzalez-Olmos, F. Llovell, A computational drop-in assessment of hydrofluoroethers in Organic Rankine Cycles. Energy 254, 124319 (2022)
M. Doubek, V. Vacek, SAFT equations of state for low GWP hydrofluoroethers heat transfer fluids. Int. J. Thermophys. 43, 138 (2022)
R. Span, Multiparameter Equations of State—An Accurate Source of Thermodynamic Property Data, 1st edn. (Springer, Berlin, 2000)
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, REFPROP—reference fluid thermodynamic and transport properties. NIST standard reference database 23, v.10 (2018)
T. Minamihonoki, H. Ogawa, S. Murakami, H. Nomura, Excess molar enthalpies and volumes of binary mixtures of nonafluorobutylmethylether with ethylene glycol ethers at T= 298.15 K. J. Chem. Thermodyn. 37, 1186–1195 (2005)
Product Information - 3M Novec 7100 Engineered Fluids, 7007HB. 3M Center, St. Paul (2009)
Product Information - 3M Novec 7200 Engineered Fluids, 7005HB. 3M Center, St. Paul (2009)
H.C. Knachel, V. Benin, W.E. Moddeman, J.C. Birkbeck, T.A. Kestner, T.L. Young, Characterization by NMR of reactants and products of hydrofluoroether isomers, CF3 (CF2)3OCH3 and (CF3)2C(F)CF2OCH3, reacting with isopropyl alcohol. Magn. Reson. Chem. 51, 407–413 (2013)
S.L. Outcalt, M. McLinden, Automated densimeter for the rapid characterization of industrial fluids. Ind. Eng. Chem. Res. 46, 8264–8269 (2007)
S.L. Outcalt, Calibration fluids and calibration equations: how choices may affect the results of density measurements made with u-tube densimeters. J. Res. Natl Inst. Stand. 123, 123017 (2018)
E.F. May, W.J. Tay, M. Nania, A. Aleji, S. Al-Ghafri, J.P.M. Trusler, Physical apparatus parameters and model for vibrating tube densimeters at pressures to 140 MPa and temperatures to 473 K. Rev. Sci. Instrum. 85, 095111 (2014)
T.J. Fortin, A. Laesecke, M. Freund, S. Outcalt, Advanced calibration, adjustment, and operation of a density and sound speed analyzer. J. Chem. Thermodyn. 57, 276–285 (2013)
Z. Wagner, M. Bendová, J. Rotrekl, A. Sýkorová, M. Čanji, N. Parmar, Density and sound velocity measurement by an Anton Paar DSA 5000 density meter: precision and long-time stability. J. Mol. Liq. 329, 115547 (2021)
O. Prokopová, A. Blahut, M. Čenský, M. Součková, V. Vinš, Comments on temperature calibration and uncertainty estimate of the vibrating tube densimeter operated at atmospheric pressure. J. Chem. Thermodyn. 173, 106855-1–106855-11 (2022)
Anton Paar GmbH, Reference Guide DMA 4100 M, DMA 4500 M, DMA 5000 M, Document No. XDLIB016EN-E. Anton Paar GmbH, Graz (2018)
G. Fritz, G. Scherf, O. Glatter, Applications of densiometry, ultrasonic speed measurements, and ultralow shear viscosimetry to aqueous fluids. J. Phys. Chem. B 104, 3463–3470 (2000)
M.M. Piñeiro, D. Bessières, J.L. Legido, H. Saint-Guirons, \(p\rho t\) measurements of nonafluorobutyl methyl ether and nonafluorobutyl ethyl ether between 283.15 and 323.15 K at pressures up to 40 MPa. Int. J Thermophys. 24, 1265–1276 (2003)
J. Cendón, M.M. Piñeiro, D. Bessières, J. Vijande, J.L. Legido, High-pressure densities of the binary mixture methyl nonafluorobutyl ether+ hexane. J. Chem. Eng. Data 49, 1368–1372 (2004)
N. Muñoz-Rujas, F. Aguilar, J.M. García-Alonso, E.A. Montero, Thermodynamics of binary mixtures 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane (HFE-7200)+ 2-propanol: High pressure density, speed of sound and derivative properties. J. Chem. Thermodyn. 131, 630–647 (2019)
A. Vetere, Again the Rackett equation. Chem. Eng. J. 49, 27–33 (1992)
S.L. Outcalt, A. Laesecke, T.J. Fortin, Density and speed of sound measurements of hexadecane. J. Chem. Thermodyn. 42, 700–706 (2010)
A. Aminian, D. Celný, A. Jäger, E. Mickoleit, V. Vinš, Ideal gas heat capacity and critical properties of HFE-type engineering fluids: ab initio predictions of \({c_{\rm p}^{ig}}\), modeling of phase behavior and thermodynamic properties using Peng-Robinson and volume-translated Peng–Robinson equations of state. Int. J. Thermophys. 43, 87 (2022)
Product Information - 3M Novec 7000 Engineered Fluids, 10316HB. 3M Center, Building 224-3N-11, St. Paul, MN 55144-1000 (2014)
Product Information - 3M Novec 7300 Engineered Fluids, 7003HB. 3M Center, St. Paul (2009)
Product Information - 3M Novec 7500 Engineered Fluids, 7010HB. 3M Center, St. Paul (2008)
H. Qi, D. Fang, X. Meng, J. Wu, Liquid density of HFE-7000 and HFE-7100 from T = (283 to 363) K at pressures up to 100 MPa. J. Chem. Thermodyn. 77, 131–136 (2014)
H. Ohta, Y. Morimoto, J.V. Widiatmo, K. Watanabe, Liquid-phase thermodynamic properties of new refrigerants: pentafluoroethyl methyl ether and heptafluoropropyl methyl ether. J. Chem. Eng. Data 46, 1020–1024 (2001)
Y. Zhou, E.W. Lemmon, REFPROP-Equations of state for RE245cb2, RE347mcc, RE245fa2, and R1216. to be submitted to J. Phys. Chem. Ref. Data (2018)
K. Tanaka, Measurements of vapor pressure and saturated liquid density for HFE-7100. Jpn. Soc. Refrig. Air Condition. Eng. 32, 285–291 (2015)
M.B. Shiflett, A. Yokozeki, Liquid-liquid equilibria of hydrofluoroethers and ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 52, 2413–2418 (2007)
M.L. Huber, E.W. Lemmon, PROperties of FIre Suppressant SYstems “PROFISSY”—a spreadsheet application for fire-suppressant bottle-filling calculations. NIST Techn. Note 2132, 1–82 (2021)
D. Fang, Y. Li, X. Meng, J. Wu, Liquid density of HFE-7200 and HFE-7500 from T = (283 to 363) K at pressures up to 100 MPa. J. Chem. Thermodyn. 69, 36–42 (2014)
N. Muñoz-Rujas, F. Aguilar, J.M. García-Alonso, E.A. Montero, High pressure density and speed of sound of hydrofluoroether fluid 1,1,1,2,2,3,4,5,5,5-decafluoro-3-methoxy-4-(trifluoromethyl)-pentane (HFE-7300). J. Chem. Thermodyn. 121, 1–7 (2018)
D. Sommer, R. Kleinrahm, R. Span, W. Wagner, Measurement and correlation of the \((p, \rho , t)\) relation of liquid cyclohexane, toluene, and ethanol in the temperature range from 233.15 K to 473.15 K at pressures up to 30 MPa for use as density reference liquids. J. Chem. Thermodyn. 43, 117–132 (2011)
N. Muñoz-Rujas, J.P. Bazile, F. Aguilar, G. Galliero, E. Montero, J.L. Daridon, Speed of sound and derivative properties of hydrofluoroether fluid HFE-7500 under high pressure. J. Chem. Thermodyn. 112, 52–58 (2017)
Acknowledgments
The authors would like to thank Prof. Natalia Muñoz-Rujas for discussing the results of her group.
Funding
Open access publishing supported by the National Technical Library in Prague. The study was supported by the Czech Science Foundation project no. GA22-03380S and the institutional support RVO: 61388998.
Author information
Authors and Affiliations
Contributions
VV, OP, and MR contributed to conceptualization ; OP, AB, JH, KK, MČ contributed to methodology; OP, AB, AA, MČ, JH, KK, and VV contributed to formal analysis and investigation; OP and VV contributed to writing–original draft preparation ; MR, AB, AA, and VV contributed to writing-review and editing; : VV contributed to funding acquisition; MR and VV contributed to supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Special Issue in Honor of Professor Roland Span’s 60th Birthday.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prokopová, O., Blahut, A., Hajduch, J. et al. Influence of Isomeric Composition and Sample Handling on the Liquid Density of Hydrofluorethers Measured by Vibrating Tube Densimeter at 0.1 MPa. Int J Thermophys 44, 139 (2023). https://doi.org/10.1007/s10765-023-03247-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03247-6