Abstract
The porous structure of three different, commercially available porous carbonaceous materials is investigated by the αS-plot method and by the t-plot method. Subsequently, the electrochemical properties of sulfur-free porous carbon electrodes from inspected materials are studied by cyclic voltammetry. The comparison of double-layer capacitances with the corresponding adsorption isotherms of N2 reveals the role of micropores during the capacitive charging of carbons by Li+. The studied carbons are added to the sulfur cathodes and evaluated. The cyclic voltammograms show no contribution of micropores in the carbon structure to the electrochemical processes taking place in the lithium–sulfur coin cell. The highest specific capacity of 816 mAh/g is observed for material with the lowest content of micropores in the structure (14%). The partially mesoporous and partially microporous (65%) sample and the predominantly microporous one (87%), show specific capacities of 664 mAh/g and 560 mAh/g, respectively. The galvanostatic cycling of lithium–sulfur coin cells with carbonaceous additives reveals that the mesopores and macropores in the carbon structure increase the specific charge capacity of the lithium–sulfur batteries and that the micropores improve the cycling stability of these batteries.
Graphical abstract

Similar content being viewed by others
Introduction
The rising demand for energy storage devices with high energy density leads to intensive research on new battery systems. The lithium–sulfur (Li–S) battery is a promising system in this field, due to its non-toxicity, low cost, theoretical energy density of ca. 2600 Wh/kg, and capacity of 1675 mAh/g, which overcomes the commonly used lithium–ion batteries [1,2,3]. Sulfur is an abundant, inexpensive, and environmentally friendly element. Therefore, Li–S batteries have great potential for applications in such devices as drones [4, 5] and electric vehicles [6, 7]. Despite of undeniable promising properties of the Li–S system, some challenges have to be solved to enable the spreading of Li–S batteries into practical applications. The following limitations remain to impede the successful commercialization of Li–S cells. The Li metal usually serves as an anode that represents safety issues [8]. During the discharge process, the sulfur is reduced to lithium sulfide (Li2S) at the cathode side. This process has multiple steps, in which sulfur is initially reduced to long-chain polysulfides (Li2S6–8), followed by a further reduction to form short-chain polysulfides (Li2S2–4) and final transformation to insoluble Li2S. The sulfur reduction to Li2S is connected with the volumetric expansion of the cathode and poor conductivity of both the reactant and the product [9]. Moreover, the intermediate lithium polysulfides are soluble and cause the well-known shuttle effect leading to a decline in cycle life and capacity [10]. In recent years, huge attention has been paid to all above-mentioned problematic aspects; however, some questions remain to be addressed.
Conductive carbons are widely used in various technologies and play a significant role in Li–S batteries. The above-mentioned problem of the electrically insulating nature of sulfur in the Li–S system is solved by embedding the active material into/on the conductive carbon. Various forms of carbon were widely used as cathode materials in Li–S batteries—spheres [11], nanofibers [12], nanotubes [13, 14], 3D [15, 16], and porous materials [17, 18]. Besides the improvement of cathode conductivity, the possibility of anchoring sulfur and the products of its reduction in carbon structure was studied [19, 20]. The characterization of the porous structure of carbon is crucial for a given application. According to the International Union of Pure and Applied Chemistry (IUPAC) definition, porous materials can be classified into three different categories based on pore size: microporous with pore widths smaller than 2 nm, mesoporous with pore widths 2–50 nm and macroporous with pore widths larger than 50 nm [21]. The micropores have two subgroups: ultramicropores (pore widths < 0.7 nm) and supermicropores (pore widths 0.7–2 nm). The commonly used method for pore structure characterization of carbonaceous materials is the physical adsorption of nitrogen and subsequent analysis of adsorption isotherms. Effective textural characterization of porous carbons is provided by comparative t-plot and αS-plot methods [21,22,23,24]. Another useful technique is the subtracting pore effect (SPE) method applied to a high-resolution αS-plot (based on isotherms from relative pressure 10–6–1) [25,26,27,28].
The effect of different pores in the structure of carbon on the electrochemical performance of Li–S batteries has been widely discussed recently, but the role of carbon micropores in the cathode material is not yet fully clarified [2, 29,30,31,32,33,34,35]. Our current work aims at further elucidation of this issue. We report here a detailed study of the porous structure of three different, commercially available porous carbonaceous materials by subtracting pore effect method applied to a high-resolution αS-plot and by the t-plot method. These three carbons are used as sulfur-free cathodes and as additives in the sulfur cathodes and their electrochemical behavior is studied to evaluate the effect of micro-/meso-/macropores of carbon material on the electrochemical performance of a corresponding cathode.
Results and discussion
Material characterization
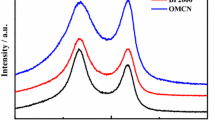
The powder materials CFluka, CPenta, and CTOB (see “Experimental” for details) were characterized by Raman spectroscopy (Fig. 1). Two main Raman features of carbonaceous materials are observed in the spectra of all studied samples. The first one centered at ca. 1325 cm−1 belongs to the D-band, which is connected with the breathing mode and requires disorder or defect in the sp2 carbon network for its activation [33, 36,37,38,39,40]. The D mode is dispersive, i.e. its frequency scales linearly with the photon excitation energy. The second band, the G-band at ~ 1590 cm−1, arises from the symmetrical stretching mode of graphitic domains with sp2 hybridization. The decoupled D to G peak intensity ratio (denoted as ID/IG) can be considered as a straightforward indicator of disorder in sp2 systems, i.e. this ratio can point to differences in the graphitization degrees of carbon materials. The calculated intensity ratio ID/IG for the three studied carbonaceous materials are 1.23, 1.04, and 1.28 for CFluka, CPenta, and CTOB, respectively. The ID/IG ratios of CTOB and CFluka are similar and indicate more disordered structures and a higher number of defects in these materials compared to CPenta. The lower ratio for CPenta shows a possibly higher degree of graphitization than for CTOB and CFluka.
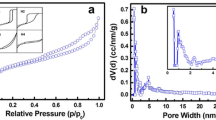
All carbon materials were investigated by nitrogen adsorption at 77 K for the relative pressures P/P0 from 10–6 to 1. Figure 2 shows the adsorption isotherms of CFluka, CPenta, and CTOB. The studied powders have different porous structures. The adsorption isotherm of CFluka corresponds to type I according to the IUPAC classification with a sharp knee and high uptakes at relatively low pressures characteristic for microporous materials. The adsorption isotherm of CPenta shows the features of type I and type IV isotherms. It exhibits significant nitrogen adsorption at low pressures indicating the presence of narrow micropores (pore width < 2 nm) and hysteresis loop at P/P0 higher than 0.4 indicating the presence of mesopores in the structure. The CTOB adsorption isotherm has a shape close to type II isotherm, which is normally associated with the external surface of non-porous material or with macroporous powders. The N2 adsorption at low P/P0 can indicate a minor contribution of micropores in structure. Table 1 shows the calculated BET specific surface areas for all our samples. The CFluka material has the highest specific surface area 1039 m2/g. The CPenta has a BET surface area comparable with CFluka (1028 m2/g). On the other hand, the CTOB sample has a five times lower specific surface area compared to the former.
Subsequently, the adsorption isotherms were analyzed by a comparative method of high-resolution αS-plots. Wang et al. [25] tested six different non-graphitized carbon blacks as non-porous reference adsorbents for the αS-plot analysis of porous carbons. Their carbon denoted as CB-1 (Monarch 280, Cabot Corporation) had the best properties for use as a standard in comparative αS-plot analysis [25]. Therefore, the reference data from the N2 (77.4 K) adsorption isotherms of Monarch 280 (Table S2 in Ref. [25]) were used as a standard αS-curve for the construction of αS-plots of our studied samples (CFluka, CPenta, and CTOB). The final high-resolution αS-plots of CFluka, CPenta, and CTOB are shown in Fig. 3. The αS-plots of CFluka and CPenta have two upward swings for αS < 1 (below micropore saturation). These swings are a consequence of the adsorption in micropores. The first swing for αS < 0.5 (P/P0 < 0.007) is called the filling swing and it is connected with monolayer adsorption in micropores enhanced by overlapping adsorption potentials of micropore walls [21, 25, 27]. The second upward swing at αS between 0.5 and 1 is observed in the case the larger micropores (width > 1 nm [26]) are present in the carbon structure. This upward adsorption enhancement is the effect of continuous micropore filling in the remaining space of the micropore after the monolayer adsorption and it is called the cooperative swing [26, 27] or sometimes the condensation swing [28, 41]. There is a plateau of N2 adsorption for CFluka in the region of micropores saturation (αS > 1) (see Fig. 3). On the other hand, the plateau for sample CPenta is visible at higher αS compared to CFluka and the plot corresponds to the CPenta isotherm in Fig. 2 with obvious capillary condensation in mesopores. The αS-plot observed for CTOB material evidences a high rate of linearity (see Fig. 3). Generally, the linearity of the αS-plot corresponds to unrestricted monolayer-multilayer adsorption typical for non-porous or macroporous solids [21]. Only the slight upward shifts for αS < 0.5 and αS > 1 indicate some content of micropores and mesopores, respectively, in the CTOB structure. The material producer (TOB New Energy) declares the CTOB to be a porous material with a pore diameter of about 130–150 nm (i.e. macropores), but their mercury intrusion porosimetry tests revealed intrusion peak in the range of pore sizes 20–200 nm. This agrees with our observed almost linear shape of the CTOB αS-plot (see Fig. 3). The SPE method was applied to high-resolution αS-plots, and the total surface areas of studied powders were determined from the slope of the linear fit of αS-plot passing through 0 and αS ≈ 0.5. The difference between the BET surface area and the total surface area determined from the αS-plot for the CTOB sample is negligible. On the other hand, the αS-plot total surface areas of CFluka and CPenta are slightly higher (8% and 6%, respectively) compared to corresponding BET surface areas (see Table 1). The reason for this inconsistency is that the BET surface area of CFluka and CPenta, even with the use of Rouquerol’s criteria [42], is underestimated due to insufficient monolayer adsorption of nitrogen on micropores [25, 41]. Kaneko et al. introduced the external surface (surface out of micropores and mesopores) estimation from the almost plateau region of αS-plot at high values of αS [27]. This procedure was used for the determination of the external surface of our studied porous carbonaceous materials. Figure 3 shows the fits of respective αS-plots. The fitted region for CTOB was chosen across all the αS values outside the micropore region, due to the high linearity of CTOB αS-plot, which is typical for non-porous/macroporous samples, where most of the surface area is external or in macropores. Table 1 summarizes the surface area analysis by αS-plot method and the surface areas out of micropores (Sout of mic) determined by t-plot analysis. The data show the highest percentage of micropores (SBET − Sout of mic; 87%) in the structure of the CFluka material. The CPenta has an external surface area comparable with CFluka but the contribution of micropores to the overall surface area is lower (65%). On the other hand, the CTOB sample has low amount of micropores in the structure (14%).
The αS-plots for CPenta (top, blue), CFluka (middle, red), and CTOB (bottom, black) materials. Dotted lines: the linear fits passing through the origin were used for total surface areas determination. Dashed lines: the linear fits for estimation of external surface areas (color version available online)
Electrochemical performance
Recently, the carbon pores and primarily micropores in the cathode material and their effect on the electrochemical performance of Li–S batteries have been widely discussed. There is still a lack of clarity on their role [2, 29,30,31, 33, 34] and the influence on capacity and stability [2, 29, 30, 32, 35]. Therefore, we focused on the electrochemical behavior of carbonaceous materials CFluka, CPenta, and CTOB in detail.
First, the electrochemical properties of sulfur-free porous carbon electrodes CFluka, CPenta, and CTOB were studied by cyclic voltammetry, where 40% of carbon C65 was added to electrodes for conductivity improvement (for details see “Experimental”). The cyclic voltammograms were carried out at scan rates 10, 5, 2, and 1 mV/s in potential window from 2 to 3 V vs Li/Li+. Figure 4 shows cyclic voltammograms of CFluka, CPenta, CTOB and pure C65 at a scan rate of 5 mV/s. The cyclic voltammograms of CFluka and CTOB have an expected rectangular shape typical for capacitive charging. The CPenta exhibited quasi-rectangular voltammograms for scan rates 2 mV/s and lower. The low contribution of C65 to the overall cyclic voltammetry response of CFluka, CPenta, and CTOB is evident from Fig. 4. The double layer capacitance (CDL) was determined from cyclic voltammetry data according to Eq. (1):
where Ia (Ic) is the anodic (cathodic) current at a fixed potential and ν is the scan rate. Table 2 summarizes the double layer capacitances per gram of material calculated for each material and each scan rate from current responses at potential 2.5 V. The CFluka and CPenta have at least two times higher CDL compared to CTOB for scan rates of 5 mV/s and slower. It agrees with the data of total surface areas Stot in Table 1, where the CTOB has the lowest total surface area (206 m2/g) and CFluka has the highest one (1127 m2/g), but close to Stot of CPenta (1085 m2/g). However, the Stot of CFluka and CPenta contains a high contribution of surface area in micropores (87% and 65%, respectively) compared to CTOB (14%, see Table 1). Therefore, the data of CDL in Table 2 indicate, that the micropores play a role in carbon capacitive charging by Li+. Almost negligible changes of CDL for CTOB and CFluka at different scan rates are evident from Table 2. These data indicate solely capacitive current’s contribution to the overall current response of these carbonaceous materials. The CDL increase is observed for slower scan rates of CPenta carbon. This can be caused by the more complex porous structure of the material, which is the mixture of a high percentage of micropores and mesopores.
Second, the CFluka, CPenta, and CTOB were studied as additives in the sulfur composite cathode in the 2032-coin cells containing Li anode, and a glass microfiber separator. The coin cells were investigated by cyclic voltammetry and by galvanostatic chronopotentiometry. Cyclic voltammetry represents an analytic tool providing information about the charge capacity and the processes taking place during the electrochemical reaction. Figure 5 shows the cyclic voltammograms (2nd scan) of the CFluka, CPenta, and CTOB composite cathodes in a Li–sulfur cell measured at the scan rate of 0.1 mV/s. The cyclic voltammograms exhibit two reduction peaks at the potentials near 2.34 V and 2.02 V, and one oxidation peak at ca. 2.40 V. The first cathodic peak at 2.34 V is assigned to the reduction of S8 to polysulfides Li2Sx (3 ≤ x ≤ 8), and the second cathodic peak near 2.02 V is assigned to subsequent reductions up to Li2S2 and Li2S. A single oxidation peak at 2.4 V for CFluka and CPenta is observed due to the sluggish kinetics of the solid–liquid two-phase oxidation from the insoluble L2S or Li2S2 to short-/long-chain polysulfides, which causes an overlap of the corresponding peak in the oxidation branch of the cyclic voltammogram with that corresponding to oxidation of long-chain polysulfides to sulfur. The doublet of anodic peaks is distinguishable for CTOB only. The specific charge capacities of CFluka, CPenta, and CTOB composite cathodes calculated from the oxidation (charge) branch of the cyclic voltammogram are 560 mAh/g, 664 mAh/g, and 816 mAh/g, respectively. The CTOB, consisting mainly of wide pores, evinces the highest specific capacity. Conversely, both CFluka and CPenta with high contribution of micropores in the structures have lower specific capacities than CTOB. This trend is inverse as compared to the case of double-layer capacities from cyclic voltammograms of CFluka, CPenta, and CTOB in sulfur-free electrochemical cells discussed above (see Fig. 4 and Table 2).
The corresponding coin cells were subsequently studied by galvanostatic cycling. The electrochemical performance of the sulfur composite cathodes with CFluka, CPenta, or CTOB was studied by galvanostatic chronopotentiometry at the charging/discharging rate of 0.1 C. Figure 6 shows the galvanostatic curves of the CFluka, CPenta, or CTOB sulfur cathode during 60 charge/discharge cycles. The CTOB sulfur cathode exhibits the largest capacities but also the largest decrease in capacity during 60 cycles. The partially mesoporous and partially microporous CPenta (see Table 1) exhibits lower capacities than CTOB and good stability over 60 cycles. There is an evident trend of specific charge capacity increase with an increasing percentage of mesopores/macropores in the carbon structure in Fig. 6, which is caused by the conservation of polysulfides in the meso/macropores during the charging/discharging process and suppressing their shuttle effect. On the contrary, the cathode with microporous CFluka exhibited the lowest capacities after the first 10 cycles, but stable electrochemical performance during further cycling. Interestingly, the CFluka and CTOB with similar content of defects in the structure (see Fig. 1) have very different trends in stability during cycling. Electrochemical data indicate that carbonaceous additives with wider pores (mesopores/macropores) and a more opened surface provide higher specific charge capacities in a sulfur composite cathode than microporous ones. Hence, mesoporous/microporous carbons represent the optimum additive for sulfur composite cathode in Li–S batteries. However, the more complex structure of CTOB exhibiting wide pore size distribution (including 14% of micropores) manifests itself by structural instability during 60 days charging/discharging due to its inability to buffer the volume changes during the formation of soluble polysulfides (discharging) and backward deposition of sulfur (charging). The structural changes result in a pronounced capacity drop during cycling. On the other side, the structure of microporous CFluka is stable during 60 cycles, but exhibits lower specific capacity despite its high specific surface area. The observed low capacity and the fact that most of the specific surface area of CFluka is located inside the micropores (see Table 1) prove the low participation of micropores in the electrochemical reduction of sulfur and polysulfides oxidation, which is opposite to sulfur-free electrochemical cells discussed above (see Fig. 4 and Table 2). This conclusion agrees with the observed cyclic voltammogram of CFluka in Fig. 5. The low size of micropores (pore widths < 2 nm) represents a spatial constraint on sulfur accommodation. Li et al. observed the current peak with a maximum at 1.7 V for specially prepared sulfur-microporous carbon composite material and this peak was assigned to the solid–solid process, the reduction of smaller sulfur molecules confined within the micropores [43]. However, there is no additional current increase observed at potentials near 1.7 V for the cyclic voltammogram of CFluka in Fig. 5. This can also explain the good stability of CFluka and CPenta during cycling. The sulfur composite cathode employing materials with a high percentage of micropores in the structure (CFluka/CPenta) does not stress the micropores during charging/discharging by volumetric changes. This represents a significant stabilization factor. Indeed, the CPenta exhibits nearly no capacity decay over 60 cycles in contrast to CTOB. The majority of its surface area in micropores seems to compensate for the effect of disintegration during cycling. In summary, mesopores and particularly macropores in the carbon structure increase the specific charge capacity of the cathodes in the coin cell due to a spatial constraint of polysulfides near the sulfur composite cathode. On the other hand, micropores suppress carbon structure disintegration during cycling and thus improve the stability of the sulfur composite cathode.
Galvanostatic chronopotentiometry curves measured at 0.1 C of Li–S cells containing sulfur composite cathodes with CTOB (top, black circles), CPenta (middle, blue circles), and CFluka (bottom, red circles). Charging curves are denoted by full circles, and discharging curves by open circles. The coulombic efficiencies are depicted by empty diamonds (CTOB, black), empty squares (CPenta, blue), and crosses (CFluka, red) (color version available online)
Conclusion
Three commercially available porous carbon materials (CFluka, CPenta, and CTOB) were studied in detail by subtracting pore effect method applied to a high-resolution αS-plot and by t-plot method to characterize their porous structures. The CFluka has the highest specific surface area 1039 m2/g and is found to be a primarily microporous material with the highest ratio of micropores (87%) in the structure as compared to CPenta and CTOB. The CPenta is a mesoporous/microporous material with a specific surface area comparable with CFluka (1028 m2/g). The contribution of micropores to the overall surface area is lower (65%). On the other hand, the CTOB sample contains a minority of micropores in the structure (14%) and exhibited N2 adsorption isotherm typical for non-porous or macroporous materials. Raman spectroscopy proved highly defective structures of all carbonaceous samples.
The electrochemical behavior of carbonaceous materials CFluka, CPenta, and CTOB was studied in detail to evaluate the effect of micro-/meso-/macroporosity of the carbonaceous material on the electrochemical performance of corresponding cathodes. The electrochemical properties of sulfur-free porous carbon electrodes CFluka, CPenta, and CTOB were studied by cyclic voltammetry. The double-layer capacities of CTOB and CFluka for different scan rates indicate solely capacitive charging of these carbonaceous materials. The CDL increase was observed for slower scan rates of CPenta carbon, which can be caused by the more complex porous structure of the material, which is the mixture of a high percentage of micropores and mesopores. Moreover, the comparison of CDL data with corresponding total surface areas of CFluka, CPenta, and CTOB reveals the appreciable role of micropores during capacitive charging of carbons by Li+. To study the effect of carbonaceous additives on the electrochemical performance of a corresponding sulfur composite cathode, the CFluka, CPenta, and CTOB were added to the sulfur cathodes and evaluated by cyclic voltammetry and galvanostatic chronopotentiometry. The cyclic voltammograms reveal a standard electrochemical behavior of these cathodes with no contribution of micropores in electrochemical processes. The highest specific capacity of 816 mAh/g is observed for CTOB. The partially mesoporous CPenta shows a specific capacity of 664 mAh/g and the microporous CFluka 560 mAh/g. The cycling by galvanostatic chronopotentiometry reveals excellent stability for both micropores-containing materials: CFluka and CPenta. The mesopores and mainly macropores in the carbon structure increase the specific charge capacity of the Li–S batteries based on electrolyte 1 M LiTFSI DOL/DME (1:1 v/v) by spatial constraint of polysulfides near the sulfur composite cathode. On the contrary, micropores improve the stability of the Li–sulfur battery by hindering the carbon structure disintegration during cycling.
Experimental
The cathodes for sulfur-free electrochemical tests were prepared as follows: The carbon (Fluka—Activated Charcoal (CFluka), Penta—Activated Carbon (CPenta), or TOB New Energy—TOB-HPC (CTOB)) was mixed in an agate mortar with the conductive carbon black C65 (Timcal) and 2% aqueous solution of carboxymethylcellulose (Sigma) added and mortared again. The final mass ratio of the carbon to C65 and carboxymethylcellulose was 5:4:1. Subsequently, the viscous paste was created by adding deionized water into the mixture and deposited on the aluminum mesh by dip coating. The final Al mesh was dried at 50 °C under a vacuum overnight and the electrodes were stored under an Ar atmosphere in a glove box.
The cathodes for Li–S coin cells were prepared by a procedure reported previously by Zlamalova et al. [44] Briefly, carbon CFluka, CPenta, or CTOB was mixed with sulfur (Sigma-Aldrich) in a mass ratio of 1:4 and mortared in an agate mortar for 20 min. Subsequently, the mixture was heated at 155 °C for 15 h under an Ar atmosphere in a Teflon container and mortared again. The conductive carbon black C65 (Timcal) and 2% aqueous solution of carboxymethylcellulose (Sigma) were added and mortared. The final mass ratio of the carbon/sulfur composite to C65 and carboxymethylcellulose was 7:2:1. The as-prepared cathode material contained 56 wt% of sulfur. The concentration of sulfur was verified by X-ray fluorescence spectroscopy (XRF) analysis reported previously [45]. Subsequently, the viscous paste was created by adding deionized water to the mixture. The resulting slurry was stirred at 400 RPM overnight and deposited on aluminum foil by doctor-blading. The coated Al foil was dried at 50 °C under a vacuum overnight and cut into disc electrodes of 15 mm in diameter. The areal sulfur loading was adjusted to 0.5–0.6 mg/cm2. The electrodes were stored under an Ar atmosphere in a glove box. The electrodes were assembled in 2032 coin-type test cells with Li-foil anode (14 mm in diameter), glass microfiber separator (Whatman), and 20 mm3 of electrolyte.
The electrolyte for sulfur-free electrochemical tests and Li–S coin cells tests was identical. It consisted of 1.0 M lithium bis-(trifluoromethanesulfonyl) imide (LiTFSI, Aldrich) dissolved in a mixture of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) (1:1 by volume) with 1.0 wt% LiNO3 (Aldrich) as an electrolyte-additive. LiTFSI was dried in a vacuum at 130 °C overnight, LiNO3 at 50 °C in a vacuum overnight, and the mixture of organic solvents was dried over a molecular sieve 4 Å (Aldrich). Electrolytes, solvents, and redox-active molecules were of standard quality (p.a. or electrochemical grade) purchased from Aldrich or Merck.
Raman spectra were measured on the MicroRaman system (LabRAM HR spectrometer, Horiba Jobin–Yvon) with an Olympus BX microscope. The spectra were excited by a He–Ne laser (633 nm). The Raman spectrometer was calibrated using the F1g line of Si at 520.5 cm−1. The intensities of the D and G-band were determined by fitting the spectra with a Lorentzian function. The adsorption isotherms of nitrogen were measured with ASAP 2020 apparatus (Micromeritics) at 77 K. Before the adsorption measurement, each sample was degassed at 300 °C for at least 3 h under a pressure less than 0.7 Pa. After the free space was determined at 77 K with helium the samples were evacuated for an additional 3 h at 250 °C. The surface area was determined by the BET (Brunauer, Emmett, Teller) equation. The BET surface areas of carbonaceous materials containing micropores in the structure have been calculated using Rouquerol’s criteria [42]. The surface area out of micropores was determined by t-plot analysis (thickness equation—Carbon Black STSA). Sulfur-free electrochemical measurements were carried out in a one-compartment cell using Autolab 302N apparatus (Metrohm) controlled by Nova software in the potential window from 2 to 3 V vs Li/Li+. The reference and counter electrodes were from Li metal; hence, all potentials were quoted against the reference electrode in this medium. The electrochemical measurements were carried out under an argon atmosphere in a glove box. The Li–S coin cell tests were carried out with Autolab 302N apparatus (Metrohm) controlled by Nova and Nova Battery SW in 2032 coin-type test cells at a potential window of 1.7–2.9 V vs. Li+/Li. Galvanostatic chronopotentiometry was measured in the 2032 coin-type test cells by the Neware Battery Testing System controlled by BTS 7.6 SW.
Data availability
Data will be available from the corresponding author upon request.
References
Ould Ely T, Kamzabek D, Chakraborty D, Doherty MF (2018) ACS Appl Energy Mater 1:1783
Eftekhari A, Kim DW (2017) J Mater Chem A 5:17734
Jiang JC, Fan QN, Liu HK, Chou SL, Konstantinov K, Wang JZ (2021) ACS Appl Mater Inter 13:28405
Cleaver T, Kovacik P, Marinescu M, Zhang T, Offer G (2018) J Electrochem Soc 165:A6029
Dorfler S, Althues H, Hartel P, Abendroth T, Schumm B, Kaskel S (2020) Joule 4:539
Yin YX, Xin S, Guo YG, Wan LJ (2013) Angew Chem Int Ed 52:13186
Mi K, Jiang Y, Feng JK, Qian YT, Xiong SL (2016) Adv Funct Mater 26:1571
Lin DC, Liu YY, Cui Y (2017) Nat Nanotechnol 12:194
Zhu W, Paolella A, Kim CS, Liu D, Feng Z, Gagnon C, Trottier J, Vijh A, Guerfi A, Mauger A, Julien CM, Armandd M, Zaghib K (2017) Sustain Energy Fuels 1:737
Peng HJ, Huang JQ, Cheng XB, Zhang Q (2017) Adv Energy Mater 7:1700260
Wang XZ, Zhang S, Zhang HY, Gao S, Han SJ, Xu QY, Xu JJ, Lu W, Wu XD, Chen LW (2019) ACS Sustain Chem Eng 7:17491
Luo C, Niu SZ, Zhou GM, Lv W, Li BH, Kang FY, Yang QH (2016) Chem Commun 52:12143
Jeong YC, Lee K, Kim T, Kim JH, Park J, Cho YS, Yang SJ, Park CR (2016) J Mater Chem A 4:819
Ding YL, Kopold P, Hahn K, van Aken PA, Maier J, Yu Y (2016) Adv Funct Mater 26:1112
Zhu X, Ye JW, Lu YF, Jia XL (2019) ACS Sustain Chem Eng 7:11241
Nguyen QH, Luu VT, Lim SN, Lee YW, Cho YY, Jun YS, Seo MH, Ahn W (2021) ACS Appl Mater Inter 13:28036
Nitze F, Fossum K, Xiong SZ, Matic A, Palmqvist AEC (2016) J Power Sources 317:112
Hencz L, Wu ZZ, Zheng MT, Bat-Erdene M, Qian SS, Su Z, Gu XX, Liu XH, Zhang SQ, Chen H (2022) ACS Appl Energy Mater 5:4991
Zou YW, Guo DY, Yang B, Zhou L, Lin PR, Wang JY, Chen XA, Wang S (2021) ACS Appl Mater Inter 13:50093
Li X, Li XF, Banis MN, Wang BQ, Lushington A, Cui XY, Li RY, Sham TK, Sun XL (2014) J Mater Chem A 2:12866
Rouquerol F, Rouquerol J, Sing KSW, Llewellyn P, Maurin G (2014) Adsorption by powders and porous solids principles, methodology and applications. Academic Press, Oxford
Lippens BC, Linsen BG, Deboer JH (1964) J Catal 3:32
Deboer JH, Linsen BG, Osinga TJ (1965) J Catal 4:643
Lowell S, Shields JE, Thomas MA, Thommes M (2004) Characterization of porous solids and powders: surface area, pore size and density. Kluwer Academic Publishers, Dordrecht
Wang SW, Vallejos-Burgos F, Furuse A, Yoshikawa Y, Tanaka H, Kaneko K (2021) Carbon 175:77
Setoyama N, Suzuki T, Kaneko K (1998) Carbon 36:1459
Kaneko K, Ishii C, Kanoh H, Hanzawa Y, Setoyama N, Suzuki T (1998) Adv Colloid Interface Sci 76–77:295
Kaneko K, Ishii C, Ruike M, Kuwabara H (1992) Carbon 30:1075
Borchardt L, Oschatz M, Kaskel S (2016) Chem Eur J 22:7324
Zhang SS (2013) J Power Sources 231:153
Kensy C, Schwotzer F, Dorfler S, Althues H, Kaskel S (2021) Batteries Supercaps 4:823
Yu CH, Yen YJ, Chung SH (2021) Nanomaterials 11:1518
Kensy C, Leistenschneider D, Wang S, Tanaka H, Dorfler S, Kaneko K, Kaskel S (2021) Batteries Supercaps 4:612
Choi YS, Park GO, Kim KH, Kwon Y, Huh J, Kim JM (2021) Chem Commun 57:10500
Tonoya T, Ando H, Takeichi N, Senoh H, Kojima T, Hinago H, Matsui Y, Ishikawa M (2023) J Phys Chem C 127:10038
Ferrari AC, Robertson J (2000) Phys Rev B 61:14095
Ferrari AC (2007) Solid State Commun 143:47
Tuinstra F, Koenig JL (1970) J Chem Phys 53:1126
Cancado LG, Jorio A, Ferreira EHM, Stavale F, Achete CA, Capaz RB, Moutinho MVO, Lombardo A, Kulmala TS, Ferrari AC (2011) Nano Lett 11:3190
Smith MW, Dallmeyer I, Johnson TJ, Brauer CS, McEwen JS, Espinal JF, Garcia-Perez M (2016) Carbon 100:678
Kaneko K, Ishii C (1992) Colloid Surf 67:203
Rouquerol J, Llewellyn P, Rouquerol F (2006) Stud Surf Sci Catal 160:49
Li Z, Jiang Y, Yuan LX, Yi ZQ, Wu C, Liu Y, Strasser P, Huang YH (2014) ACS Nano 8:9295
Zlamalova M, Pitna Laskova B, Vinarcikova M, Zukalova M, Kavan L (2022) J Solid State Electrochem 26:639
Zukalová M, Vinarčíková M, Pitňa Lásková B, Kavan L (2023) Mater Chem Phys 296:127246
Acknowledgements
This research was funded by the Grant Agency of the Czech Republic (contract no. 20-03564S).
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pitňa Lásková, B., Zukalová, M., Vinarčíková, M. et al. Influence of carbon structure/porosity on the electrochemical performance in Li–sulfur batteries. Monatsh Chem 155, 353–360 (2024). https://doi.org/10.1007/s00706-023-03160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03160-6