Abstract
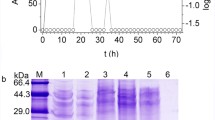
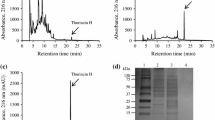
A novel Bacillus thuringiensis (Bt) bacteriocin BtCspB, active against a food-borne pathogen Bacillus cereus, was identified and purified by a traditional four-step chromatographic process with low yield (44.5 µg/L) in our lab previously. The aim of this study was to dramatically increase its yield by heterologous expression of BtCspB. The BtCspB gene from Bt BRC-ZYR2 was successfully heterologously expressed in Escherichia coli BL21 (DE3). Affinity chromatography was used to obtain the pure BtCspB up to 20 mg/L. The purified BtCspB showed a MIC value of 12.5 µg/mL and a MBC value of 50.0 µg/mL against Bacillus cereus ATCC 10987. The bacteriocin activity of BtCspB against B. cereus ATCC 10987 was further directly detected in a gel-overlay assay. The anti-B. cereus activity, however, was lower than the bacteriocin purified by the traditional four-step chromatographic process probably because of structural modifications. Compared with the traditional method, the yield of the bacteriocin by heterologous expression increased by 449 times, and the purification step was dramatically simplified, which laying a foundation for the industrial production of this novel cold-shock protein-like bacteriocin BtCspB active against B. cereus.



Similar content being viewed by others
References
Abriouel H, Franz CM, Ben Omar N, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35:201–232
Chehimi S, Pons A-M, Sable S, Hajlaoui M-R, Limam F (2010) Mode of action of thuricin S, a new class IId bacteriocin from Bacillus thuringiensis. Can J Microbiol 56:162–167
Chen H, Tian F, Li S, Xie Y, Zhang H, Chen W (2012) Cloning and heterologous expression of a bacteriocin sakacin P from Lactobacillus sakei in Escherichia coli. Appl Microbiol Biotechnol 94:1061–1068
Cutler P (1996) Size-Exclusion Chromatography. In: Doonan S (ed) Protein Purification Protocols. Method in Molecular Biology™, 59. Humana Press, New York, pp 255–267
de la Fuente-Salcido NM, Casados-Vázquez LE, Barboza-Corona JE (2013) Bacteriocins of Bacillus thuringiensis can expand the potential of this bacterium to other areas rather than limit its use only as microbial insecticide. Can J Microbiol 59:515–522
Farhangnia L, Ghaznavi-Rad E, Mollaee N, Abtahi H (2014) Cloning, expression, and purification of recombinant lysostaphin From Staphylococcus simulans. Jundishapur J Microbiol 7:e10009
Field D, Cotter PD, Hill C, Ross RP (2015) Bioengineering lantibiotics for therapeutic success. Front Microbiol 6:1363
Gibbs GM, Davidson BE, Hillier AJ (2004) Novel expression system for large-scale production and purification of recombinant class IIa bacteriocins and its application to piscicolin 126. Appl Environ Microbiol 70:3292–3297
Gray EJ, Lee KD, Souleimanov AM, Di Falco MR, Zhou X, Ly A, Charles TC, Driscoll BT, Smith DL (2006) A novel bacteriocin, thuricin 17, produced by plant growth promoting rhizobacteria strain Bacillus thuringiensis NEB17: isolation and classification. J Appl Microbiol 100:545–554
Huang T, Xiao Y, Pan J, Zhang L, Gelbič I, Guan X (2015) Characterization of cry1Cb3 and cry1Fb7. from Bacillus thuringiensis subsp galleriae. Open Life Sci 10:521–528
Huang T, Zhang X, Pan J, Su X, Jin X, Guan X (2016) Purification and characterization of a novel cold shock protein-like bacteriocin synthesized by Bacillus thuringiensis. Sci Rep 6:35560
Jiang H, Li P, Gu Q (2016) Heterologous expression and purification of plantaricin NC8, a two-peptide bacteriocin against Salmonella spp. from Lactobacillus plantarum ZJ316. Protein Expr Pur 127:28–34
Kamoun F, Mejdoub H, Aouissaoui H, Reinbolt J, Hammami A, Jaoua S (2005) Purification, amino acid sequence and characterization of Bacthuricin F4, a new bacteriocin produced by Bacillus thuringiensis. J Appl Microbiol 98:881–888
Kamoun F, Ben Fguira I, Tounsi A, Abdelkefi-Mesrati L, Sanchis V, Lereclus D, Jaoua S (2009) Generation of mini-Tn10 transposon insertion mutant library of Bacillus thuringiensis for the investigation of genes required for its bacteriocin production. FEMS Microbiol Lett 294:141–149
Karakas-Sen A, Narbad A (2012) Heterologous expression and purification of NisA, the precursor peptide of lantibiotic nisin from Lactococcus lactis. Acta Biol Hung 63:301–310
Lee H, Churey JJ, Worobo RW (2009) Biosynthesis and transcriptional analysis of thurincin H, a tandem repeated bacteriocin genetic locus, produced by Bacillus thuringiensis SF361. FEMS Microbiol Lett 299:205–213
Meng F, Zhu X, Lu F, Bie X, Lu Z (2017) Functional analysis of plantaricin E and its mutant by heterologous expression in Escherichia coli. Appl Biochem Biotechnol 182:311–323
Metelev M, Serebryakova M, Ghilarov D, Zhao Y, Severinov K (2013) Structure of microcin B-like compounds produced by Pseudomonas syringae and species specificity of their antibacterial action. J Bacteriol 195:4129–4137
Montalbán-López M, van Heel AJ, Kuipers OP (2017) Employing the promiscuity of lantibiotic biosynthetic machineries to produce novel antimicrobials. FEMS Microbiol 41:5–18
Moon G-S, Pyun Y-R, Kim WJ (2006) Expression and purification of a fusion-typed pediocin PA-1 in Escherichia coli and recovery of biologically active pediocin PA-1. Int J Food Microbiol 108:136–140
O’Connor PM, Ross RP, Hill C, Cotter PD (2015) Antimicrobial antagonists against food pathogens: a bacteriocin perspective. Curr Opin Food Sci 2:51–57
Repka LM, Chekan JR, Nair SK, van der Donk WA (2017) Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem Rev 117:5457–5520
Ross PR, Morgan S, Hill C (2002) Preservation and fermentation: past, present and future. Int J Food Microbiol 79:3–16
Sahoo TK, Jena PK, Patel AK, Seshadri S (2015) Purification and Molecular Characterization of the novel highly potent bacteriocin TSU4 produced by Lactobacillus animalis TSU4. Appl Biochem Biotechnol 177:90–104
Ugras S, Sezen K, Kati H, Demirbag Z (2013) Purification and characterization of the bacteriocin thuricin Bn1 produced by Bacillus thuringiensis subsp kurstaki Bn1 isolated from a hazelnut pest. J Microbiol Biotechnol 23:167–176
Verdon J, Girardin N, Marchand A, Hechard Y, Berjeaud JM (2013) Purification and antibacterial activity of recombinant warnericin RK expressed in Escherichia coli. Appl Microbiol Biotech 97:5401–5412
Walton HF (1968) Ion exchange chromatography. Anal Chem 40:51R–62R
Wannun P, Piwat S, Teanpaisan R (2014) Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe 27:17–21
Acknowledgements
The authors thank Gale A. Kirking at English Editorial Services, s.r.o. for language correction and additional assistance in improving the text. The authors thank Xiaoyu Su, the teachers and the students of the Biopesticide Research Center for their help.
Funding
This project was supported by the National Key R&D Program of China (No. 2017YFD0200400; 2017YFE0121700; 2017YFE0122000), National Natural Science Foundation of China (No. 31672084), Fuzhou Health and Family Planning Science and Technology Project (No. 2018-S-wt7) and by institutional support RVO: 60077344 of the Biology Centre CAS, Institute of Entomology.
Author information
Authors and Affiliations
Contributions
XJ, JY, HF, YC carried out the experiments; XJ wrote the draft manuscript; IG, TH, JP, LZ, XP edited the manuscript; TH, XG designed the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, X., Yao, J., Fan, H. et al. Heterologous expression and purification of BtCspB, a novel cold-shock protein-like bacteriocin from Bacillus thuringiensis BRC-ZYR2. World J Microbiol Biotechnol 35, 23 (2019). https://doi.org/10.1007/s11274-019-2595-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2595-z