Abstract
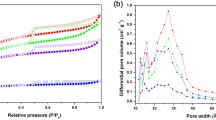
This paper presents an investigation of CO2 capture ability of ordered carbon (CMK-3) composed of a two-dimensional hexagonal array of turbostratic carbon rods of 19.7 nm and 8.7 nm in diameter with interstitial mesopores and non-restricted micropores in the rods at atmospheric pressure. The CMK-3 carbon had a mesopore mean inner diameter of 3.42 nm and a microporous structure with modus of pore diameter of 1.30 nm. Despite the fact that the mesopores did not directly take part in CO2 adsorption at atmospheric pressure, the carbon material exhibited good performance in CO2 capture, adsorbing 1.70 mmol g−1 and 1.07 mmol g−1 in the micropores at 293 and 323 K, respectively. The open mesoporous channel structure with regular architecture provided easy accessibility of the micropores in the carbon walls that formed the structure of CMK-3 carbon. Enhancement of accessibility improved the molecular transport of CO2, providing advantageous kinetics compared to purely microporous materials. The adsorption rate obtained from kinetics studies were decreasing with gas pressure increase in the system and were increasing with temperature increase. The ordered mesoporous-microporous carbon structure allowed excellent reversibility, regeneration ability and high selectivity of CO2 over N2 molecules.
Graphical abstarct















Similar content being viewed by others
References
Du, Z., Nie, X., Deng, S., Zhao, L., Li, S., Zhang, Y., Zhao, J.: Comparative analysis of calculation method of adsorption isosteric heat: Case study of CO. Microporous Mesoporous Mater. 298, 110053 (2020)(b).
Jiang, S., Chen, Z., Shan, L., Chen, X., Wang, H.: Committed CO2 emissions of China’s coal-fired power generators from 1993 to 2013. Energy Policy 104, 295–302 (2017)
Lee, S.Y., Park, S.J.: A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 23, 1–11 (2015)
Ochedi, F.O., Liu, Y., Adewuyi, Y.G.: State-of-the-art review on capture of CO2 using adsorbents prepared from waste materials. Process Saf. Environ. Prot. 139, 1–25 (2020)
Morales-Ospino, R., Goltzman, Y., Torres, A.E.B., Vilarrasa-García, E., Bastos-Neto, M., Cavalcante, C.L., Azevedo, D.C.S., Marques, C.R.M., de Aquino, T.F., de Oliveira, V.R.: Assessment of the potential use of zeolites synthesized from power plant fly ash to capture CO2 under post-combustion scenario. Adsorption 26, 1153–1164 (2020)
Villarreal, A., Garbarino, G., Riani, P., Finocchio, E., Bosio, B., Ramírez, J., Busca, G.: Adsorption and separation of CO2 from N2-rich gas on zeolites: Na-X faujasite vs Na-mordenite. J. CO2 Util. 19, 266–275 (2017).
Cecilia, J.A., Vilarrasa-García, E., Morales-Ospino, R., Bastos-Neto, M., Azevedo, D.C.S., Rodríguez-Castellón, E.: Insights into CO2 adsorption in amino-functionalized SBA-15 synthesized at different aging temperature. Adsorption 26, 225–240 (2020)
Liu, Y., Lin, X., Wu, X., Liu, M., Shi, R., Yu, X.: Pentaethylenehexamine loaded SBA-16 for CO2 capture from simulated flue gas. Powder Technol. 318, 186–192 (2017)
Landaverde-Alvarado, C., Morris, A.J., Martin, S.M.: Gas sorption and kinetics of CO2 sorption and transport in a polymorphic microporous MOF with open Zn (II) coordination sites. J. CO2 Util. 19, 40–48 (2017).
Mohamedali, M., Henni, A., Ibrahim, H.: Investigation of CO2 capture using acetate-based ionic liquids incorporated into exceptionally porous metal–organic frameworks. Adsorption 25, 675–692 (2019)
Lee, S., Filburn, T.P., Gray, M., Park, J.W., Song, H.J.: Screening test of solid amine sorbents for CO2 capture. Ind. Eng. Chem. Res. 47, 7419–7423 (2008)
Zhang, G., Zhao, P., Xu, Y.: Development of amine-functionalized hierarchically porous silica for CO2 capture. J. Ind. Eng. Chem. 54, 59–68 (2017)
Li, X., Bai, S., Zhu, Z., Sun, J., Jin, X., Wu, X., Liu, J.: Hollow carbon spheres with abundant micropores for enhanced CO2 adsorption. Langmuir 33, 1248–1255 (2017)(b).
Ma, R., Hao, J., Chang, G., Wang, Y., Guo, Q.: Nitrogen-doping microporous adsorbents prepared from palm kernel with excellent CO2 capture property. Can. J. Chem. Eng. 98, 503–512 (2020)
Vorokhta, M., Morávková, J., Řimnáčová, D., Pilař, R., Zhigunov, A., Švábová, M., Sazama, P.: CO2 capture using three-dimensionally ordered micromesoporous carbon. J. CO2 Util. 31, 124–134 (2019).
Majchrzak, A., Nowak, W.: Separation characteristics as a selection criteria of CO2 adsorbents. J. CO2 Util. 17, 69–79 (2017).
Russell, B., Migone, A.: Low temperature adsorption study of CO2 in ZIF-8. Microporous Mesoporous Mater. 246, 178–185 (2017)
Mukherjee, A., Okolie, J.A., Abdelrasoul, A., Niu, C., Dalai, A.K.: Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. (China) 83, 46–63 (2019)
Jarczewski, S., Drozdek, M., Michorczyk, P., Cuadrado-Collados, C., Gandara-Loe, J., Silvestre-Albero, J., Kuśtrowski, P.: Oxidative dehydrogenation of ethylbenzene over CMK-1 and CMK-3 carbon replicas with various mesopore architectures. Microporous Mesoporous Mater. 271, 262–272 (2018)
Kim, H.S., Kang, M.S., Lee, S., Lee, Y.W., Yoo, W.C.: N-doping and ultramicroporosity-controlled crab shell derived carbons for enhanced CO2 and CH4 sorption. Microporous Mesoporous Mater. 272, 92–100 (2018)
Ammendola, P., Raganati, F., Chirone, R.: CO2 adsorption on a fine activated carbon in a sound assisted fluidized bed: Thermodynamics and kinetics. Chem. Eng. J. 322, 302–313 (2017)
Parshetti, G.K., Chowdhury, S., Balasubramanian, R.: Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 148, 246–254 (2015)
Reid, C.R., Thomas, K.M.: Adsorption of gases on a carbon molecular sieve used for air separation: Linear adsorptives as probes for kinetic selectivity. Langmuir 15, 3206–3218 (1999)
Cao, S., Zhao, H., Hu, D., Wang, J.A., Li, M., Zhou, Z., Shen, Q., Sun, N., Wei, W.W.: Preparation of potassium intercalated carbons by in-situ activation and speciation for CO2 capture from flue gas. J. CO2 Util. 35, 59–66 (2020).
Du, J., Li, W., Ren, Z., Guo, L., Lu, A.: Synthesis of mechanically robust porous carbon monoliths for CO2 adsorption and separation. J. Energy Chem. 42, 56–61 (2020)(a).
Majchrzak-Kucȩba, I., Wawrzyńczak, D., Ściubidło, A., Zdeb, J., Smółka, W., Zajchowski, A.: Stability and regenerability of acivated carbon used for CO2 removal in pilot DR-VPSA unit in real power plant conditions. J. CO2 Util. 29, 1–11 (2019).
Wu, T., Dong, J., De France, K., Zhang, P., Zhao, X., Zhang, Q.: Porous carbon frameworks with high CO2 capture capacity derived from hierarchical polyimide/zeolitic imidazolate frameworks composite aerogels. Chem. Eng. J. 395, 124927 (2020).
Li, D., Tian, Y., Li, L., Li, J., Zhang, H.: Production of highly microporous carbons with large CO2 uptakes at atmospheric pressure by KOH activation of peanut shell char. J. Porous Mater. 22, 1581–1588 (2015)
Wang, S., Xu, Y., Miao, J., Liu, M., Ren, B., Zhang, L., Liu, Z.: Facile synthesis of microporous carbon xerogels for highly selective CO2 adsorption. J. Clean. Prod. 253, 120023 (2020).
Donald Carruthers, J., Petruska, M.A., Sturm, E.A., Wilson, S.M.: Molecular sieve carbons for CO2 capture. Microporous Mesoporous Mater. 154, 62–67 (2012)
Silvestre-Albero, J., Wahby, A., Sepúlveda-Escribano, A., Martínez-Escandell, M., Kaneko, K., Rodríguez-Reinoso, F.: Ultrahigh CO2 adsorption capacity on carbon molecular sieves at room temperature. Chem. Commun. (Camb) 47, 6840–6842 (2011)
Li, K., Tian, S., Jiang, J., Wang, J., Chen, X., Yan, F.: Pine cone shell-based activated carbon used for CO2 adsorption. J. Mater. Chem. A. 4, 5223–5234 (2016)
Wu, Y.J., Yang, Y., Kong, X.M., Li, P., Yu, J.G., Ribeiro, A.M., Rodrigues, A.E.: Adsorption of pure and binary CO2, CH4, and N2 gas components on activated carbon beads. J. Chem. Eng. Data. 60, 2684–2693 (2015)
Yoo, H.-M., Lee, S.-Y., Park, S.-J.: Ordered nanoporous carbon for increasing CO2 capture. J. Solid State Chem. 197, 361–365 (2013)
Teague, C.M., Schott, J.A., Stieber, C., Mann, Z.E., Zhang, P., Williamson, B.R., Dai, S., Mahurin, S.M.: Microporous and hollow carbon spheres derived from soft drinks: Promising CO2 separation materials. Microporous Mesoporous Mater. 286, 199–206 (2019)
Rahimi, M., Babu, D.J., Singh, J.K., Yang, Y.B., Schneider, J.J., Muller-Plathe, F.: Double-walled carbon nanotube array for CO2 and SO2 adsorption. J. Chem. Phys. 143, 124701 (2015).
Hwang, C., Jin, Z., Lu, W., Sun, Z., Alemany, L.B., Jay, R.: In situ synthesis of polymer-modified mesoporous carbon CMK-3 composites for CO2 sequestration. ACS Appl. Mater. Interfaces 3, 4782–4786 (2011)
Montagnaro, F., Silvestre-Albero, A., Silvestre-Albero, J., Rodríguez-Reinoso, F., Erto, A., Lancia, A., Balsamo, M.: Post-combustion CO2 adsorption on activated carbons with different textural properties. Microporous Mesoporous Mater. 209, 157–164 (2015)
Zhou, J., Su, W., Sun, Y., Deng, S., Wang, X.: Enhanced CO2 sorption on ordered mesoporous carbon CMK-3 in the presence of water. J. Chem. Eng. Data 61, 1348–1352 (2016)
Anbia, M., Salehi, S.: Synthesis of polyaniline/mesoporous carbon nanocomposites and their application for CO2 sorption. J. Polym. Res. 23, 1–9 (2016)
Sevilla, M., Fuertes, A.B.: CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 366, 147–154 (2012)
Lakhi, K.S., Cha, W.S., Choy, J.H., Al-Ejji, M., Abdullah, A.M., Al-Enizi, A.M., Vinu, A.: Synthesis of mesoporous carbons with controlled morphology and pore diameters from SBA-15 prepared through the microwave-assisted process and their CO2 adsorption capacity. Microporous Mesoporous Mater. 233, 44–52 (2016)
Chandrasekar, G., Son, W.J., Ahn, W.S.: Synthesis of mesoporous materials SBA-15 and CMK-3 from fly ash and their application for CO2 adsorption. J. Porous Mater. 16, 545–551 (2009)
Nguyen, C., Do, D.D.: The Dubinin-Radushkevich equation and the underlying microscopic adsorption description. Carbon 39, 1327–1336 (2001)
Lippens, B.C., de Boer, J.H.: Studies on pore systems in catalysts: V. The t method. J. Catal. 4, 319–323 (1965)
Medek, J., Weishauptová, Z., Kovář, L.: Combined isotherm of adsorption and absorption on coal and differentiation of both processes. Microporous Mesoporous Mater. 89, 276–283 (2006)
Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G.: Adsorption by powders and porous solids: principles, methodology and applications. Elsevier (2014).
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Barrett, E.P., Joyner, L.G., Halenda, P.P.: The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 73, 373–380 (1951)
Horri, N., Sanz-Pérez, E.S., Arencibia, A., Sanz, R., Frini-Srasra, N., Srasra, E.: Effect of acid activation on the CO2 adsorption capacity of montmorillonite. Adsorption 26, 793–811 (2020)
Tzabar, N., ter Brake, H.J.M.: Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride. Adsorption 22, 901–914 (2016)
Al-Ghouti, M.A., Da’ana, D.A.: Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 393, 122383 (2020)
Cen, Q., Fang, M., Wang, T., Majchrzak-Kucęba, I., Wawrzyńczak, D., Luo, Z.: Thermodynamics and regeneration studies of CO2 adsorption on activated carbon. Greenh. Gases Sci. Technol. 6, 787–796 (2016)
Brandani, S.: Kinetics of liquid phase batch adsorption experiments. Adsorption 27, 353–368 (2020)
Dopita, M., Emmel, M., Salomon, A., Rudolph, M., Matêj, Z., Aneziris, C.G., Rafaja, D.: Temperature evolution of microstructure of turbostratic high melting coal-tar synthetic pitch studied using wide-angle X-ray scattering method. Carbon 81, 272–283 (2015)
Dopita, M., Rudolph, M., Salomon, A., Emmel, M., Aneziris, C.G., Rafaja, D.: Simulations of X-ray scattering on two-dimensional, graphitic and turbostratic carbon structures. Adv. Eng. Mater. 15, 1280–1291 (2013)
Warren, B.E.: X-ray diffraction in random layer lattices. Phys. Rev. 59, 693 (1941)
Warren, B.E., Bodenstein, P.: The diffraction pattern of fine particle carbon blacks. Acta Crystallogr. 18, 282–286 (1965)
Cantini, P., Boato, G., Salvo, C., Tatarek, R., Terreni, S.: Graphite surface mean square displacements at low temperature studied by He atoms scattering. Phys B+C. 108, 955–956 (1981)
Schmidt, W.: Calculation of XRD patterns of simulated FDU-15, CMK-5, and CMK-3 carbon structures. Microporous Mesoporous Mater. 117, 372–379 (2009)
Ostafiychuk, B.K., Mandzyuk, V.I., Kulyk, Y.O., Nagirna, N.I.: SAXS investigation of nanoporous structure of thermal-modified carbon materials. Nanoscale Res. Lett. 9, 160 (2014)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquérol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985)
Burwell, R.L.: Manual of symbols and terminology for physicochemical quantities and units, appendix II: Definitions, terminology and symbols in colloid and surface chemistry. Pure Appl. Chem. 46, 71–90 (1976)
Kruk, M., Jaroniec, M., Kim, T.W., Ryoo, R.: Synthesis and characterization of hexagonally ordered carbon nanopipes. Chem. Mater. 15, 2815–2823 (2003)
Jain, S.K., Pellenq, R.J.-M., Gubbins, K.E., Peng, X.: Molecular modeling and adsorption properties of ordered silica-templated CMK mesoporous carbons. Langmuir 33, 2109–2121 (2017)
Rehman, A., Park, S.J.: Environmental remediation by microporous carbon: An efficient contender for CO2 and methylene blue adsorption. J. CO2 Util. 34, 656–667 (2019).
Martín-Martínez, J.M., Torregrosa-Maciá, R., Mittelmeijer-Hazeleger, M.C.: Mechanisms of adsorption of CO2 in the micropores of activated anthracite. Fuel 74, 111–114 (1995)
Srenscek-Nazzal, J., Narkiewicz, U., Morawski, A.W., Wróbel, R., Gesikiewicz-Puchalska, A., Michalkiewicz, B.: Modification of commercial activated carbons for CO2 adsorption. Acta Phys. Pol. A. 129, 394–401 (2016)
Burri, H., Anjum, R., Gurram, R.B., Mitta, H., Mutyala, S., Jonnalagadda, M.: Mesoporous carbon supported MgO for CO2 capture and separation of CO2/N2. Korean J. Chem. Eng. 36, 1482–1488 (2019)
Tao, D.J., Mao, F.F., Luo, J.J., Zhou, Y., Li, Z.M., Zhang, L.: Mesoporous N-doped carbon derived from tea waste for high-performance CO2 capture and conversion. Mater. Today Commun. 22, 100849 (2020).
Mukhtar, A., Mellon, N., Saqib, S., Khawar, A., Rafiq, S., Ullah, S., Al-Sehemi, A.G., Babar, M., Bustam, M.A., Khan, W.A., Tahir, M.S.: CO2/CH4 adsorption over functionalized multi-walled carbon nanotubes; an experimental study, isotherms analysis, mechanism, and thermodynamics. Microporous Mesoporous Mater. 294, 109883 (2020).
Arif, A.F., Kobayashi, Y., Schneider, E.M., Hess, S.C., Balgis, R., Izawa, T., Iwasaki, H., Taniguchi, S., Ogi, T., Okuyama, K., Stark, W.J.: Selective low-energy carbon dioxide adsorption using monodisperse nitrogen-rich hollow carbon submicron spheres. Langmuir 34, 30–35 (2018)
Montiel-Centeno, K., Barrera, D., Villarroel-Rocha, J., Moreno, M.S., Sapag, K.: Hierarchical nanostructured carbons as CO2 adsorbents. Adsorption 25, 1287–1297 (2019)
Serafin, J., Narkiewicz, U., Morawski, A.W., Wróbel, R.J., Michalkiewicz, B.: Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 18, 73–79 (2017).
Phuriragpitikhon, J., Ghimire, P., Jaroniec, M.: Tannin-derived micro-mesoporous carbons prepared by one-step activation with potassium oxalate and CO2. J. Colloid Interface Sci. 558, 55–67 (2019)
Liu, L., Deng, Q.-F., Ma, T.-Y., Lin, X.-Z., Hou, X.-X., Liu, Y.-P., Yuan, Z.-Y.: Ordered mesoporous carbons: citric acid-catalyzed synthesis, nitrogen doping and CO2 capture. J. Mater. Chem. 21, 16001–16009 (2011)
Yang, Z., Guo, X., Zhang, G., Xu, Y.: One-pot synthesis of high N-doped porous carbons derived from a N-rich oil palm biomass residue in low temperature for CO2 capture. Int. J. Energy Res. 2, 1–13 (2020)
Chiang, Y.-C., Wu, C.-Y., Chen, Y.-J.: Effects of activation on the properties of electrospun carbon nano fibers and their adsorption performance for carbon dioxide. Sep. Purif. Technol. 233, 116040 (2020).
Mawunya Kutorglo, E., Kovačovič, J., Trunov, D., Hassouna, F., Fučíková, A., Kopecký, D., Sedlářová, I., Šoóš, M.: Preparation of carbon-based monolithic CO2 adsorbents with hierarchical pore structure. Chem. Eng. J. 388, 124308 (2020).
Jang, E., Choi, S.W., Hong, S.M., Shin, S., Lee, K.B.: Development of a cost-effective CO2 adsorbent from petroleum coke via KOH activation. Appl. Surf. Sci. 429, 62–71 (2018)
Hinkov, I., Lamari, F.D., Langlois, P., Dicko, M., Chilev, C., Pentchev, I.: Carbon dioxide capture by adsorption (review). J. Chem. Technol. Metall. 51, 609–626 (2016)
Yuan, B., Wu, X., Chen, Y., Huang, J., Luo, H., Deng, S.: Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 47, 5474–5480 (2013)
Li, D., Zhou, J., Zhang, Z., Li, L., Tian, Y., Lu, Y., Qiao, Y., Li, J., Wen, L.: Improving low-pressure CO2 capture performance of N-doped active carbons by adjusting flow rate of protective gas during alkali activation. Carbon 114, 496–503 (2017)(a).
He, S., Chen, G., Xiao, H., Shi, G., Ruan, C., Ma, Y., Dai, H., Yuan, B., Chen, X., Yang, X.: Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 582, 90–101 (2021)
Wang, J., Yang, J., Krishna, R., Yang, T., Deng, S.: A versatile synthesis of metal–organic framework-derived porous carbons for CO2 capture and gas separation. J. Mater. Chem. A. 4, 19095–19106 (2016)
Deng, S., Hu, B., Chen, T., Wang, B., Huang, J., Wang, Y., Yu, G.: Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 21, 125–133 (2015)
Wang, J., Huang, L., Yang, R., Zhang, Z., Wu, J., Gao, Y., Wang, Q., O’Hare, D., Zhong, Z.: Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 7, 3478–3518 (2014)
Sarker, A.I., Aroonwilas, A., Veawab, A.: Equilibrium and kinetic behaviour of CO2 adsorption onto zeolites, carbon molecular sieve and activated carbons. Energy Procedia. 114, 2450–2459 (2017)
Everett, D.H.: Thermodynamics of adsorption. III. Analysis and discussion of experimental data. Trans. Faraday Soc. 46, 957–969 (1950).
Vorokhta, M., Švábová, M., Weishauptová, Z.: CO2 kinetic sorption rate study dependence on pressure and concentration step. 16th Int. Multidiscip. Sci. GeoConference SGEM. 3, 107–114 (2016).
Hong, S.-M., Jang, E., Dysart, A.D., Pol, V.G., Lee, K.B.: CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 6, 34590 (2016)
Zhang, Z., Zhang, W., Chen, X., Xia, Q., Li, Z.: Adsorption of CO2 on zeolite 13X and activated carbon with higher surface area. Sep. Sci. Technol. 45, 710–719 (2010)
Soares, J.L., Oberziner, A.L.B., Jose, H.J., Rodrigues, A.E., Moreira, R.F.P.M.: Carbon dioxide adsorption in Brazilian coals. Energy Fuels 21, 209–215 (2007)
Acknowledgements
This work was supported by the research organization RVO: 67985891, the project NanoCent—Nanomaterials Centre for Advanced Applications (Project No. CZ.02.1.01/0.0/0.0/15_003/0000485), financed by European Regional Development Fund (ERDF), and the Research Infrastructure NanoEnviCz (Project No. LM2018124 and CZ.02.1.01/0.0/0.0/18_046/0015586), supported by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that they are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vorokhta, M., Morávková, J., Dopita, M. et al. Effect of micropores on CO2 capture in ordered mesoporous CMK-3 carbon at atmospheric pressure. Adsorption 27, 1221–1236 (2021). https://doi.org/10.1007/s10450-021-00322-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-021-00322-y