Abstract
A polymer probe based on N-(2-hydroxypropyl)methacrylamide copolymers labelled with a fluorescent dye Dy-633 or Cy-7 and decorated with targeting oligopeptides GE-7 or GE-11, specific targeting ligands binding to epidermal growth factor receptor (EGFR) highly expressed on surface of tumour cells, was designed, synthesised and characterised. Specific accumulation of the polymer probe in the tumour mass is a prerequisite for successful fluorescence-guided endoscopic surgery as the fluorescence signal from the malignant cells enables more precise resection of the tumour without damaging the healthy tissue. Flow cytometry and confocal microscopy was used to assess the binding efficacy of the oligopeptide conjugates to EGFR on the cell membranes of the malignant cells. The results showed that the highest binding efficacy was achieved with polymers bearing the GE-11 targeting oligopeptide in human EGFR-positive hypopharyngeal carcinoma cells (FaDu) and in breast adenocarcinoma cells (MDA-MB-231). Similarly, the polymer probes targeted by the GE-11 oligopeptidewere found in vivo as highly effective in tumour accumulation, as determined from fluorescence imaging. Indeed, the ex vivo cross-section of the tumours showed significant tumour border fluorescence proving the potential of the studied polymer probes. Moreover, the presence of the active targeting moiety on the polymer-drug conjugate should enable the use of such a conjugate as a targeted polymer system for treatment of solid tumours. Replacement of the fluorescent probe with a cytostatic drug provides a targeted polymer nanocancerostatic for advanced treatment of neoplastic diseases, thus the polymer probes have multiple functions.
Export citation and abstract BibTeX RIS
Introduction
Tumours in the head and neck area are the sixth most frequent oncological pathology, with head and neck squamous cell carcinomas (HNSCC) representing approximately 95% of malignancies in this area. The increasing incidence of HSNCC has been recognised worldwide, with more than 600 000 cases per year, of which, the most common are tumours of the oral cavity, pharynx, and oropharynx [1]. These are responsible for more than 300 000 deaths annually [2]. Smoking, especially in combination with alcohol intake and human papillomavirus (HPV) infection, is the prime risk factor for HNSCC in oropharynx.
Surgery remains the best therapeutic treatment option for HNSCCs in comparison with radiotherapy and chemotherapy, despite recent advances in both these therapeutic approaches. Currently, the minimisation of secondary surgery damage, that is, injury caused by unwanted resection of non-cancerous tissues together with the cancerous tissue, is one of main interests of practical surgery to reduce the cosmetic and functional postoperative consequences (e.g. swallowing problems, articulation, and voice formation). At the same time, the surgery should be radical enough to completely remove all the malignant tissue while the necessary, non-cancerous borders of resection are maintained. Indeed, radical and contemporary precise resection of the entire tumour mass would provide more opportunity for healing, thus avoiding any revision interventions in the case of their recurrence or persistence, which both have a negative impact, not only on the patient themselves, but also represent a high public health insurance burden.
One such approaches endoscopic surgery, whose indications and technological possibilities are presently expanding. However, in the context of oncological endoscopic diagnostics or surgery, it is still problematic and nowadays even crucial, to determine the actual margins of the tumour tissue. There are possibilities for tumour marking [3] using imaging methods such as narrow band imaging (NBI) [4] or autofluorescence of tumour tissues [5], but these methods have limited reliability and can only be used for some types of tumours, either those in specific locations or those situated close to mucosal surfaces.
A marking system based on a high molecular fluorescent polymer probe is currently under development, which after intravenous application, would allow tracing the edge of a tumourous tissue more precisely, thereby useful for guiding the surgeon during the operation [6, 7]. The fluorescently labelled tumour borders would be then easily observed using a CCD camera or an endoscope fluorescence detection system. A high molecular weight polymeric conjugate is used to reduce the rate of the renal excretion, thus prolonging the circulation of the probe in the body, as increased accumulation of the polymer system is a prerequisite for successful fluorescence-guided endoscopic surgery. The targeting unit can be attached to the same polymer chain simultaneously with the dye or even with a drug.
We conducted a preclinical study of one such guiding system based on a polymer carrier prepared by reversible addition-fragmentation chain transfer (RAFT) copolymerisation of N-(2-hydroxypropyl) methacrylamide (HPMA) and a reactive co-monomer. The resulting polymer precursors have a narrow molecular weight distribution and the fluorescent dye is attached with a targeting peptide, specifically targeting receptors over expressed in tumour cells. In this work, we focused on the epidermal growth factor receptor (EGFR), which is highly expressed on the surface of tumourous HNSCC cells [8]. The oligopeptides GE-7 (NPVVGYIGERPQYRDL) and GE-11 (YHWYGYTPQNVI) were used as the targeting ligand as their affinity to EGFR was repeatedly reported in past [9, 10]. To compare the effectivity of the respective probes, a control polymer without any targeting peptide and conjugates with scrambled peptide sequences were also synthesised and evaluated to compare their EGFR binding activity with that of the peptide-targeted polymer probes.
Experimental methods
Materials and methods
1-Aminopropan-2-ol, 2,2'-azobis-(isobutyronitrile) (AIBN), N,N'-diisopropylcarbodiimide (DIC), N,N-dimethylformamide (DMF), ethyldiisopropylamine (DIPEA), 1-hydroxybenzotriazole (HOBt), methacryloyl chloride, piperidine, trifluoroacetic acid (TFA), triisopropylsilane (TIPS), and all other reagents and solvents were purchased from Sigma-Aldrich (Czech Republic). TentaGel Rink amide resin, ethyl cyano(hydroxyimino)acetate (Oxyma), (benzotriazol-1-yloxy)-trispyrrolidinophosphonium hexafluorophosphate (PyBOP), 9-fluorenylmethoxycarbonyl (Fmoc)-amino acid derivatives and 1-(9-fluorenylmethyloxycarbonyl)amino-3,6,9,12,15,18,21,24,27,30,33,36-dodecaoxanonatriacontan-39-oic acid (Fmoc-Peg12-COOH) were purchased from Iris Biotech, GmbH, Germany. 5-Azidopentanoic acid was obtained from Bachem, Germany, and amino-1-(11,12-didehydrodibenzo[b,f]azocin-5(6H)-yl)propan-1-one (Dbco-NH2) was purchased from Click Chemistry Tools. Amino derivatives of fluorescent dyes Cyanine 7 (Cy-7-NH2) and Dyomics-633 (Dy-633-NH2) were obtained from Lumiprobe, GmbH, Germany and Dyomics GmbH, Germany. All amino acids were L-configuration. Methacryloyl chloride, 1-aminopropan-2-ol, and dichloromethane were distilled immediately before use. All chemicals and solvents were analytical grade. Solvents were purified and dried using standard procedures. Monitoring of the peptide purity and conjugation of the peptide to the reactive copolymer was performed by HPLC using column Chromolith Performance RP-18e, 100 × 4.6 mm (Merck, Germany), and a linear gradient of water-acetonitrile, 0%–100% acetonitrile in the presence of 0.1% TFA with a UV–vis diode array detector (Shimadzu, Japan). Determination of the molecular weights and polydispersities of the copolymers was performed by size exclusion chromatography (SEC) on a HPLC system (Shimadzu) equipped with refractive index, UV, and multiangle light scattering DAWN 8 EOS (Wyatt Technology Corp., Santa Barbara, CA) detectors using a TSK 3000 SWXL column (Tosoh Bioscience, Japan) and 80% methanol, 20% 0.3 M acetate buffer pH 6.5 at a flow rate of 0.5 ml min−1. The calculation of molecular weights from the light scattering detector was based on the known injected mass, assuming 100% mass recovery. The content of thiazolidine-2-thione (TT) groups was determined spectrophotometrically on a Helios Alpha UV/vis spectrophotometer (Thermospectronic, UK) using the molar absorption coefficient for TT in methanol, ε305 = 10 280 L mol−1 cm−1. Determination of dyes Dy-633 or Cy-7 was also determined spectrophotometrically using the molar absorption coefficient for Dy-633 (Cy-7) in methanol, ε637 = 159 000 L mol−1 cm−1 (ε750 = 199 000 L mol−1 cm−1). Peptide sequences were assembled by automatic solid phase peptide synthesis using a Liberty Blue microwave peptide synthesiser (CEM, Matthews, NC, USA), starting from the C-terminus using standard Fmoc procedures with the consecutive addition of the N-Fmoc-protected amino acid derivative (2.5 equiv.), DIC (2.5 equiv.) as an activator and Oxyma (2.5 equiv.) as an activator base, all in DMF. After attaching the last N-Fmoc-protected amino acid and removing the Fmoc, the Fmoc-PEG12-COOH (2.5 equiv.) was added, followed by the removal of Fmoc and final attachment of 5-azidopentanoic acid (2.5 equiv.) to form the azide-peptide derivatives using PyBOP (2.5 equiv.) as an activator and DIPEA (5 equiv.) as an activator base. The scrambled versions of the peptides, GE-7scr (LNEPRDIGVYRYPVQG), GE-11scr (VYHNIPYGYTQW) and GE-11scr2 (QGTHIYYNWPYV) were prepared by the same procedure as the original peptides GE-7 and GE-11. The amino acid analysis of the hydrolysed samples (6 M HCl, 115 °C, 18 h in a sealed ampule) was performed on a Chromolith Performance RP-18e reversed-phase column, 100 × 4.6 mm (Merck, Germany) using pre-column derivatisation with phthalaldehyde and 3-sulphanylpropanoic acid (excitation at 229 nm, emission at 450 nm) and gradient elution with 10%–100% of solvent B for 35 min at flow rate of 1.0 ml min−1 (solvent A: 0.05 M sodium acetate buffer, pH 6.5; solvent B: 300 ml of 0.17 M sodium acetate and 700 ml of methanol). MALDI TOF spectroscopy was performed on a Bruker Biflex III mass spectrometer.
Syntheses of monomers and polymers
HPMA was prepared through the reaction of methacryloyl chloride with 1-aminopropan-2-ol in dichloromethane [11]. 3-Methacrylamidopropanoic acid (Ma-β-Ala-OH) was synthesised by the reaction of methacryloyl chloride with 3-aminopropanoic acid in aqueous alkaline medium [12]. 3-(3-Methacrylamidopropanoyl)thiazolidine-2-thione (Ma-β-Ala-TT) was prepared by the reaction of Ma-β-Ala OH with 4,5-dihydrothiazole-2-thiol in the presence of 4-dimethylaminopyridine. The synthesis was performed as described in reference [13] using 1-ethyl-3-(3-dimethylamino-propyl)carbodiimide hydrochloride instead of N,N'-dicyclohexylcarbodiimide, allowing removal of the water-soluble urea derivative by extraction of the organic solution with water. The monomer was characterised using HPLC and ESI MS (calculated 258.3, found 259.1 M + H).
Polymer precursor 1, poly(HPMA-co-Ma-β-Ala-TT), was prepared by RAFT polymerisation of HPMA (92 mol%, 3 g) and Ma-β-Ala-TT (8 mol%, 471 mg) using AIBN (9.35 mg) as an initiator and 4-cyano-4- thiobenzoylsulphanylpentanoic acid (25.20 mg) as a chain transfer agent as described earlier [14]. The molecular weight performed by SEC was Mw = 42 000 g mol−1 and Ð = 1.14. The content of TT groups determined by UV–vis spectroscopy was 8.8 mol%.
Polymer probes with peptides were prepared by a three-step procedure. First, polymer 1 (100 mg, 57.5 μmol TT) was reacted with Dbco-NH2 (2 mol%, 3.6 mg) in 1 ml of DMA as shown in scheme
Scheme 1. Synthesis of polymer probes
Download figure:
Standard image High-resolution imageTable 1. Characterisation of prepared polymer precursor and polymer probes.
| Sample | Peptide | Peptidea wt% | Dye | Dyeb wt% |
|---|---|---|---|---|
| 1 | n.a. | 0 | 0 | 0 |
| 2 (Ref) | n.a. | 0 | Cy-7 | 0.8 |
| 3 | GE-7 | 14.9 | Cy-7 | 0.6 |
| 4 | GE-7scr | 14.8 | Cy-7 | 0.6 |
| 5 | GE-11 | 15.0 | Cy-7 | 0.7 |
| 6 | GE-11scr | 15.2 | Cy-7 | 0.7 |
| 7 | GE-11scr2 | 15.0 | Cy-7 | 0.7 |
| 8 (Ref) | n.a. | 0 | Dy-633 | 0.8 |
| 9 | GE-7 | 15.0 | Dy-633 | 0.8 |
| 10 | GE-7scr | 15.1 | Dy-633 | 0.8 |
| 11 | GE-11 | 14.9 | Dy-633 | 0.8 |
| 12 | GE-11scr | 15.0 | Dy-633 | 0.8 |
| 13 (Ref) | n.a. | 0 | Dy-633 | 2.9 |
| 14 | GE-11 | 15.0 | Dy-633 | 3.1 |
| 15 | GE-11scr | 14.9 | Dy-633 | 2.8 |
| 16 | GE-11scr2 | 15.0 | Dy-633 | 2.8 |
aPeptide content (without Peg spacer) determined by amino acid analysis. bDyes determined by UV/VIS spectrophotometry in methanol.
Cell culture
The HeLa cell line (ATCC, Poland) was cultured in Dulbecco's modified Eagle medium (DMEM). Hypopharyngeal carcinoma cells (FaDu) from ATCC and red fluorescent protein (RFP) expressing FaDu-RFP cells (gift from Dr Tržil) were cultured in modified Eagle medium (MEM) with the addition of 1% L-glutamine and 1% NEAA (non-essential amino acids). Human breast adenocarcinoma cells (MDA-MB-231) from First Faculty of Medicine, Charles University, Prague were cultured in a combined medium (DMEM/RPMI-1640, 1:1) with the addition of 1% L-glutamine. Colorectal adenocarcinoma cells (SW620) from the Institute of Microbiology of the CAS, v. v. i., Prague, were cultured in RPMI-1640 medium. All cultivation media were supplemented with 100 U of penicillin, 100 μgml−1 streptomycin and 10% foetal bovine serum, will all cells cultivated in a humidified incubator at 37 °C with 5% CO2. The chemicals were from Life Technologies/Gibco, Czech Republic.
Western blot
Cell lysates were prepared using lysis buffer (50 mM Tris-HCl, pH 7.0, 10% glycerol, 1% SDS, 2 mM EDTA) supplemented with a protease inhibitor cocktail (Sigma-Aldrich, Czech Republic) and benzonase nuclease (purity >90%; 25 U ml−1; ThermoFischer Scientific, Czech Republic). After centrifugation (3000 × g for 10 min, 4 °C), 10 μg of total protein lysate was mixed with SDS sample buffer (72 mM Tris-HCl, pH 6.8, 3% SDS, 5% glycerol, 2.5% 2-mercaptoethanol, 0.03% bromophenol blue) and subjected to SDS-PAGE. Separated proteins were transferred onto a nitrocellulose membrane and blocked in Tris-buffered saline and Tween 20 containing 1% non-fat milk. Primary antibodies targeting EGFR (#2232) and β-tubulin (#ab6046) were purchased from Cell Signalling (Biotech, Czech Republic) and Abcam (United Kingdom), respectively. Donkey anti-rabbit HRP-conjugated secondary antibody (#ab97064; Abcam, United Kingdom) was detected with the ECL substrate (SuperSignal West Pico Plus Thermo Fisher Scientific, Czech Republic) using a PXi imaging system (Syngene, Trigon, Czech Republic).
Flow cytometry
MDA-MB-231, FaDu and SW-620 were washed with PBS and harvested with 20 mM HEPES buffer with 100 mM NaCl, 0.5% BSA and 10 mM EDTA, pH 7.4 (MDA-MB-231 and SW-620) or with 0.05% trypsin-EDTA (Thermo Fisher Scientific, Czech Republic) at 37 °C for 3 min (FaDu). Subsequently, cells were washed with 0.5% BSA in PBS (BSA/PBS) and incubated with fluorescently labelled polymer probes in the dark for 1 h at 4 °C. Prior to flow cytometry, the cells were washed once with BSA/PBS, centrifuged at 1500 rpm for 3 min and resuspended in 1 μM Sytox Blue Dead Cell Stain (Thermo Fisher Scientific, Czech Republic) to distinguish live and dead cells. All measurements were acquired using a FACSVerse™ flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and analysed using FlowJo software version 10 (Tree Star Inc., Ashland, OR).
Confocal microscopy
Internalisation of the polymer probe was observed by laser scanning confocal microscopy (LSCM) on a Olympus IX83. Cells were seeded for 24 h at a density 1.5 × 105 per confocal chamber to 500 μl cell medium and incubated for 1 h in 5% CO2 at 37 °C or 1 h at 4 °C with fluorescently labelled polymers. The amount of polymer probes added to the culture media was normalised to the peptide content (10 μgml−1), which corresponds to the final probe concentration 66.7 μg ml−1 for GE-11, GE-11scr and GE-11scr2. The final probe concentration for the sample without peptide was 56.7 μg ml−1. The cell nucleus was stained with Hoechst 33342 and CellMask™ Green (1 μgml−1, Thermo Fisher Scientific, Czech Republic) for 15 min in 37 °C and 1 h in 4 °C before imaging. After incubation, cells were washed with PBS to remove residual polymer probes which were not taken up by cells. The images were obtained on a LSCM Olympus IX83 coupled with the FV10-ASW software (Olympus, Czech Republic). The samples were scanned using the 60× oil objective Plan ApoN (1.42 numerical aperture).
Cell viability
Cytotoxicity was determined using Alamar Blue® cell viability reagent (Life Technologies, Prague, Czech Republic) in HeLa, FaDu and MDA cells according to the manufacturer's protocol. Cells were seeded in 100 μl of media in 96-well plates for 24 h at a density of 5 × 103 cells/well. The cells were subsequently incubated for 72 h in 5% CO2 at 37 °C. Then, 10 μl of Alamar Blue reagent was added to each well and incubated for 4 h in 5% CO2 at 37 °C. The fluorescence intensity was measured using a Synergy Neo plate reader (Bio-Tek, Prague, Czech Republic) with excitation at 570 nm and emission at 600 nm. The cytotoxic response of the cell lines (FaDu and MDA) to different polymer probes (samples 8–12) was measured over an equivalent concentration range of 0.004–0.5 mg ml−1 of conjugated polymers for 72 h, which corresponds to a peptide content in the range of 0.6–75 μg ml−1. Cytotoxicity was expressed as mean percentage increase relative to unexposed control ±SD. All samples were measured in triplicate in three independent experiments.
Statistical analysis
All data are shown as a mean ± SEM. One-way ANOVA followed by Dunnet's post-test analysis was performed with GraphPad Prism 5. Two-sided P values <0.05 were considered statistically significant.
In vivo pilot study
All animal experiments depicted hereinafter were performed in accordance with the Act on Experimental Work with Animals (Public Notice of the Ministry of Agriculture of the Czech Republic No. 246/1992, No. 311/1997, No. 207/2004; Decree of the Ministry of the Environment of the Czech Republic No. 117/1987; and Act of the Czech National Assembly No. 149/2004) of the Czech Republic, which is fully compatible with the corresponding European Union directives.
Harvested FaDu-RFP cells were administered subcutaneously [2 × 106 cells as a mixture with BD Matrigel™ (I.T.A.-Intertact, Ltd, Prague, Czech Republic] into the abdominal right flank of athymic nu/nu mice (obtained from Velaz, Ltd and Charles River Laboratories International, Inc., Prague, Czech Republic). When the tumours had reached a size of approximately 8 mm in diameter, mice were randomly divided into groups (n = 2). All groups were then intravenously administered with 0.3 mg of copolymer dissolved in 0.1 ml of sterile PBS. Groups were as follows: a) GE-7 or GE-11 targeted copolymer; b) GE-7scr or GE-11scr control copolymer; and reference polymer with no peptide.
Tumour accumulation
Bio-distribution of the administered Cy-7-labelled copolymers was evaluated by the fluorescence intensity of the subcutaneous pharyngeal carcinoma-bearing mice in the defined intervals of 0, 3, 24 and 48 h after administration. The mice were anaesthetised by 2% isoflurane (Aerrane, Baxter, UK) and the subcutaneous tumour fluorescence was detected by the Xtreme In Vivo Imaging System (Bruker BioSpin, Ettlingen, Germany). For imaging fluorescent dye Cy-7, the excitation filter 750 nm and emission filter 830 nm were used [15]. As a background image, a reflectance screen was used for all mice. The greyscale of images was adjusted using the Bruker Molecular Imaging (BMI) Software (Bruker BioSpin, Ettlingen, Germany). The merge and quantification of the fluorescence intensity were performed using Fiji software [16]. Regions of interest (ROI) were selected based on tumour boundaries visible in reflectance. The polymer signal in the tumours with respect to time was compared by mean fluorescence intensity. The mean fluorescence intensity in the tumours was calculated using the formula: mean fluorescence intensity = fluorescence intensity in ROI/area of ROI.
Tumour distribution
Tumours were dissected 48 h after treatment, frozen in liquid nitrogen and stored at −80 °C. Histological 50 μm sections were prepared using a cryostat (CM1520, Leica Biosystems, Wetzlar, Germany). For every tumour, 3 representative sections were prepared from regions at least 1 mm from each other. The fluorescence was detected using the methods described above.
Results and discussion
Synthesis and characterisation of polymer probes
Polymer precursor poly(HPMA-co-Ma-β-Ala-TT) was prepared by RAFT polymerisation of HPMA and reactive copolymer Ma-β-Ala-TT. The polymer exhibited a narrow molecular weight distribution with a dispersity value Ð = 1.14. Incorporation of the reactive commoner Ma-β-Ala-TT to the polymer chain enabled covalent attachment of amino group-containing compounds like Dbco-NH2 or amino-fluorescent dyes, Dy-633-NH2 or Cy-7-NH2, in the subsequent reaction step. Peptides GE-7, GE-11 and their scrambled versions were synthesised by solid phase peptide synthesis using a standard Fmoc/t-butyl strategy in an automatic microwave synthesiser. The peptides were terminated with PEG12-COOH used as a spacer between the polymer chain and the targeting peptide sequence, thus increasing the accessibility of the peptide ligand for the corresponding cell receptor. The final step of peptide synthesis was the attachment of 5-azidopentanoic acid to the N-terminus of the peptide allowing a chemo-selective copper-free click reaction with the Dbco group on the polymer (scheme
Cell viability
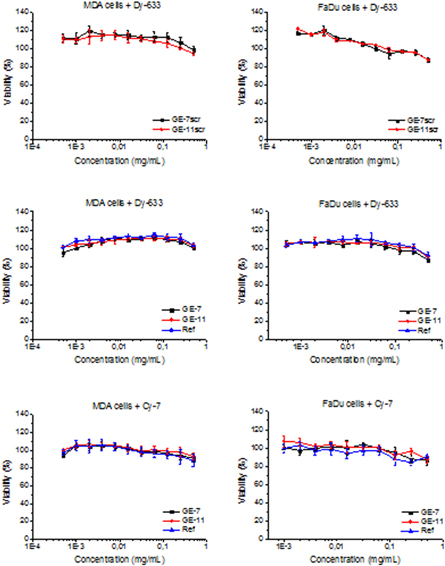
Generally, all the polymer materials should fulfill basic requirement for their application in human medicine. Such requirements consist of biocompatibility, non-immunogenicity, and stealth properties enabling minimal interaction with reticuloendothelial system and avoiding non-specific accumulation in healthy organs. The HPMA-based copolymers were evaluated successfully for their applicability recently [17]. As shown in figure 1, no significant changes in viability in all treated cells were observed after incubation with polymer-based probes, with the viability ranging from 80 to 120% of the control. There were no concentration dependent changes in viability of the cells in the studied concentration range of both the polymer probes and fluorescent dyes alone. These results indicate that all polymer probes are not cytotoxic, therefore they are suitable for use for guided surgery.
Figure 1. Cell cytotoxicity of Dy-633 labelled probes with GE-7, GE-7scr, GE-11, GE-11scr and Ref (samples 8-12) and Cy-7 labelled probe with GE-7, GE-11 and Ref (samples 3, 5 and 2) on FaDu and MDA-MB 231 cells. Probes were applied at various concentrations in a range of 0.004–0.5 mg ml−1 for 72 h in 5% CO2 at 37 °C.
Download figure:
Standard image High-resolution imageSpecific cellular binding and internalisation of polymer probes
Specific binding to EGFR, including peptide or antibody binding, is one of the current approaches for visualisation of tumour margins [18, 19]. The introduction of GE-7 or GE-11 peptide and fluorescent label Dy-633 into the HPMA polymer should enable specific ligand-mediated targeting to EGFR-positive cells overexpressed in HNSCC cells [8]. To examine the selectivity of GE-7, GE-11 and their corresponding scrambled controls (GE-7scr, GE-11scr), we evaluated their binding affinity to EGFR-positive (MDA-MB-231, FaDu) [20, 21] cells by flow cytometry (figure 2). The presence of EGFR on MDA and FaDu cells used in this study was confirmed by western blot analysis. GE-11 significantly increased the binding affinity of polymer probes in both FaDu and MDA-MB-231 cell lines, causing a 2.5 times increase in fluorescent intensity in MDA-MB-231 cells compared with control, whereas GE-7 had a minimal effect. Surprisingly, the scrambled control to GE-7 polymer probe (GE-7scr) was more efficient in binding to EGFR-positive cells than the original GE-7 containing polymer alone. A low level of non-specific binding at 4 °C was also observed for the non-targeted control polymer, probably caused by the weak interaction of the polymer structure with cell membranes.
Figure 2. EGFR binding efficacy of polymer probes containing GE-7, GE-7scr, GE-11, GE-11scr and Ref (samples 8–12) at 4 °C. The mean fluorescence intensity (MFI) values measured by flow cytometry on MDA-MB-231 (A) and FaDu (B) cells. The data are presented as the mean ± SEM of duplicates of two independent experiments. ***P < 0.001, **P < 0.01, *P < 0.05 versus the reference.
Download figure:
Standard image High-resolution imageThe GE-11 containing polymer probes were further investigated for subsequent application in vivo. Surprisingly, as the polymer probe with GE-11scr also significantly increased binding to FaDu cells compared with the reference polymer (figure 2), a new scrambled GE-11scr2 differing more noticeably in the order of amino acids from GE-11 and GE-11scr was also prepared. It was hypothesised that the binding activity of GE-11scr could be caused by similarity between the primary structure of GE-11 and GE-11scr, as they both contained the same tetrapeptide sequence YGYT. The new scrambled control for the GE-11 probe was assessed for binding of the prepared probes by flow cytometry. The results showed that the new polymer targeted by GE-11scr2 at a concentration of 100 μgml−1 had a lower binding affinity to FaDu cells than the original GE-11scr containing polymer (figure 3). To make the probes better visible using FACS, the amount of the dye was increased from 0.8 wt% to almost 3 wt%. The latter probes showed higher mean fluorescent intensity but the trend remained almost the same, the polymer probe with GE-11 showed the best binding efficiency and there was almost no difference between GE-11scr2 and the reference polymer. The GE-11-targeted probe bound significantly to MDA-MB-231 at all used concentrations (figure 3). In contrast to FaDu cells, where the increased but not statistically significant, binding affinity was visible only at 100 μg ml−1. The affinity experiment to EGFR-negative cells (SW620) [22] did not show any significant difference between the tested polymer probes at any of the concentrations tested. While there was an insignificant increase of the fluorescence intensity in EGFR-negative cells (up to 1.3 fold) using GE-11 targeted probes, we have observed more than two times higher fluorescence increase in the case of EGFR-positive cells. Thus we can conclude that the GE-11 targeted polymer nanoprobe is specific for EGFR-positive malignant cells. These results clearly demonstrated that the GE-11 targeted probe is capable to bind specifically to EGFR-positive cells.
Figure 3. Comparison of binding efficacy of polymer probes containing GE-11, GE-11scr, GE-11scr2 and Ref (samples 13–16) to EGFR positive—MDA-MB-231 (A), FaDu (B) cells and EGFR negative—SW620 (C) cells by flow cytometry. The data are presented as the mean ± SEM of duplicates of two independent experiments. ***P < 0.001, **P < 0.01, *P < 0.05 versus the reference.
Download figure:
Standard image High-resolution imageTo further investigate the cellular binding of the targeted polymer probes, a confocal microscopy experiment was performed using two different temperatures (4 °C and 37 °C) for the incubation of the polymer probes with the cancer cells. The cell nuclei were stained with the DNA binding dye Hoescht 33342 (blue) and the cytoplasm membrane was stained by CellMask™ Green (green) to better determine the subcellular localisation of the fluorescent polymer probes. Red fluorescence signal was employed to detect the presence of the polymer probes labelled with Dy-633.
Figure 4 shows confocal microscopy images of the cells (FaDu, MDA, SW620) incubated with fluorescently labelled polymer probes. After 1 h at 4 °C, significant differences in the cell-associated fluorescence of the various polymer probes could be observed. In the case of the GE-11 containing polymer probe (sample 14), the system primarily enters into the FaDu and MDA cells, where the polymers were easily detected inside the cytoplasm. Using the EGFR-negative control cells SW620, the red fluorescence signal was completely absent upon incubation with all the tested polymer probes. Incubation of the cells with the non-targeted fluorescent polymer (sample 13) at 4 °C provided no detectable red fluorescence signal (indicating negligible presence of the polymer). In contrast to the GE-11 polymer probe, GE-11scr and GE-11scr2 containing probes did not exhibit any observable internalisation into any of the tested cell lines.
Figure 4. Internalisation of fluorescently labelled polymer probes 13–16 with cells (FaDu, MDA, SW620) incubated 1 h at 4 °C was observed using confocal microscopy: conjugated polymers labelled with Dy-633 (red), nucleus stained with Hoechst 33342 (blue) and cytoplasmic membrane stained with Cell Mask Green (green).
Download figure:
Standard image High-resolution imageConfocal microscopy images of the cells (FaDu, MDA, SW620) incubated for 1 h at 37 °C with the fluorescent polymer probes are shown in figure 5. Similar to 4 °C incubation, the probe with GE-11 was internalised to the EGFR-positive cells quite rapidly. Moreover, the higher apparent red fluorescence intensities observed, even with the control polymer probes containing GE-11scr, GE-11scr2 and Ref (samples 13–15, 16), seem to indicate that all the polymer probes accumulate in cells to a larger extent at 37 °C. Interestingly, the MDA cell lines did not exhibit similar patterns of intracellular distribution of the probes as FaDu cells after 1 h incubation at 37 °C. The internalisation of the probes at 37 °C was probably mediated by increased endocytosis. Internalisation is thought to be a first-order kinetics process, where the specific rate of internalisation depends on the concentration of EGFR complexes at the cell surface [23]. Endocytic trafficking was already monitored by Sorkin using 125I-labelled EGF to measure recycling of EGF-EGFR complexes. The method is based on the observation that EGF does not significantly dissociate from EGFR in endosomes until it reaches the lysosome [24]. This recycling process seems to play an important role in the internalisation of the targeted polymer probes; it may also cause differences between the internalisation found for MDA and Fadu cells. Therefore, the influence of the endocytosis of our probes into the cells requires further study and here we hypothesise that the total cell surface area and the corresponding number of the EGF receptors are also crucial factors affecting the rate of endocytosis, which may account for the increased internalisation of the polymer probes into the MDA cells compared with FaDu cells.
Figure 5. Internalisation of fluorescently labelled polymer probes 13-16 with cells (FaDu, MDA, SW620) incubated for 1 h at 37 °C was observed using confocal microscopy: conjugated polymers labelled with Dy-633 (red), nucleus stained with Hoechst 33342 (blue) and cytoplasmic membrane stained with Cell Mask Green (green).
Download figure:
Standard image High-resolution imageAs FaDu cells grow in clusters, the internalisation process is significantly slower due to the lower cell surface area that is accessible for the polymer probes. Prolonging the incubation time increased the level of internalisation of all the polymer probes in all the tested cell lines.
The results of the confocal microscopy study clearly show that the probes accumulate mostly in cytoplasm and do not localise in the nucleus, which is in accordance with the flow cytometry data after 1 h incubation at 4 °C.
Future investigation will focus on the possible involvement of different endocytic pathways in the cellular uptake of the polymer-peptide conjugates.
In vivo tumour accumulation
The pilot in vivo study, testing the targeting efficiency of described polymeric probes for tumour imaging, was conducted using 2 mice for each tested probe. It was recently described that HPMA-based polymeric carriers of NIR Cy-7 dye can be intravitally visualised within the subcutaneous tumours for 32 days [15]. However, as the polymer fluorescence probes described here are intended for fluorescence-based navigation of the surgeon during tumour resection, a shorter period (48 h maximum) was tested. To be able to compare the accumulation of the polymeric carriers in tumours over 48 h, the tumour area was set based on the reflectance images. To ensure that the positioning of each mouse during the intravital imaging does not have an impact on the tumour area visualisation, EGFR expressing FaDu cells [25] stably expressing RFP were used, and the reflectance images were compared with the RFP signal from the tumour cells.
The course of mean fluorescent intensity of the region of interest corresponding to the tumour is depicted in figure 6. It is clear that passive accumulation through the EPR occurs for all of the tested carriers. The goal of this pilot study was to evaluate the potential of the EGFR targeting also to compare GE-7 and GE-11 peptides with regards to the unsatisfactory GE-7 results from the in vitro experiments.
Figure 6. In vivo tumour accumulation of polymer probes containing GE-7, GE-7scr, GE-11, GE-11scr and Ref (samples 2–6). The mean fluorescence intensity (MFI) was determined by the Xtreme In Vivo Imaging System. The data are presented as the mean ± SEM of duplicates of two independent experiments. * P value < 0.05.
Download figure:
Standard image High-resolution imageAll the tested probes showed preferential accumulation in the tumour tissue after 24–48 h after intravenous injection, but GE-7 targeted polymer probe (sample 3) accumulation was significantly weaker (figure 6) then accumulation of control polymer probes. These findings correspond well with the in vitro measurements (compare with figures 2 and 3). Interestingly, the GE-7 containing polymer probe accumulated in the tumour to a lesser extent than the respective control probes with GE-7scr and Ref (samples 4 and 2). As expected, all the control (not actively targeted) polymer probes containing GE-7scr or GE-11scr and Ref (samples 4, 6 and 2) demonstrated a similar tumour targeting pattern (figure 6), while the MFI values were not significantly different. Contrary to GE-7 results, GE-11 showed the largest increase in the fluorescence signal from the tumour area. Again, these findings correspond with the in vitro binding activity towards the FaDu cells (figure 3). Despite the reports that GE-7 is suitable for EGFR targeting of a polymer carrier [26, 27], our findings suggest that the GE-11 targeting peptide is more suitable for active tumour targeting of polymer imaging or delivery systems.
Due to the unsatisfactory results of the GE-7, figure 7 displays the increase in the fluorescence signal only for tumours from GE-11 targeted and Ref polymer probes (samples 2 and 5), which underwent further within-tumour distribution analysis. Generally, for the purposes of fluorescently guided surgery, targeting of the tumour margins are crucial, as this is exactly the area that surgeons need to see clearly. Ljuslinder and colleagues [28] reported that EGFR overexpressing tumour cells are more often found at the invasive margins of the tumours, rather than at the tumour stroma. Fluorescence microscopy of 50 μm thick cross sections from snap-frozen tumours collected from these groups revealed the strongest signal from the periphery of the tumours (figure 8). Interestingly, the GE-11 targeted probe showed the tumour margin more precisely than other targeted or non-targeted probes, thus proving the potential for surgery navigation. To achieve a high sensitivity of the imaging, we have selected the near-infrared dye because of the good penetration of the fluorescent signal through the tissue, which enables detection even at very low concentrations. Thus, we were able to prove the tumour accumulation and tumour-border labelling even with a relatively low dose of the polymer, 0.3 mg of polymer probe containing around 0.003 mg of dye, injected into mice proving very good sensitivity of the polymer nanoprobe.
Figure 7. Tumour targeting of polymer probe targeted with GE-11 and Ref polymer (sample 2 and 5) in mice at four different times.
Download figure:
Standard image High-resolution imageFigure 8. Fluorescence microscopy of 50 μm thick cross sections from snap-frozen tumours from three different tumours layers, A is from GE-11 containing polymer probe (sample 5) and B is from Ref polymer (sample 2).
Download figure:
Standard image High-resolution imageWe can summarize that the presented results showed the high application potential of the described polymer-based probes. The versatile concept of the polymer-dye construct enable easily scalable platform for the preparation of highly specific probes for the navigated surgery. Currently, there is onesystem using fluorescence-guided surgery clinically available. It utilizes an orally administered external fluorophore (5-aminolevulonic acid (5-ALA)).It is used mainly in neurosurgery of malignant gliomas and meningiomas and less frequently in urological cancer surgery [29]. Unfortunately, the benefit of the navigated surgery is in the case of 5-ALA rather limited due to the fact that 5-ALA is a small molecule with no active targeting to the tumour tissue. Consequently, it highlights the margins of the tumours unequivocally. We expect that similarly to 5-ALA the proposed versatile polymer nanoprobe system will be, if successfully evaluated, approved as drug type medicine, what can prolong the time of the probe to be validated for the real clinic use. We aimed to achieve sensitivities comparable to those reported in the literature for other systems for navigated surgery. Unfortunately, no useable data to make such comparison are available in the current literature. It would be useful to critically evaluate the data in a future face-to-face comparative study. Possible combination of various targeting ligands, dyes and controllable setting of the molecular weight of the construct make the described polymer platform highly novel in term of the practical application.
Conclusion
The current study described the synthesis and in vitro and in vivo evaluation of novel polymer probes designed for fluorescence-guided surgery navigation. Fluorescently labelled polymers were decorated with EGFR binding peptides, GE-7 and GE-11, to enhance the accumulation of the polymer probe in EGFR-positive tumours, especially in the tumour periphery. Polymer probes with the GE-11 EGFR targeting ligand accumulated more in EGFR-positive cells in vitro in contrast to the oligopeptide-free controls. In concordance, the significantly enhanced accumulation of the polymer probes in EGFR-positive tumours was also found in vivo, thus proving the hypothesis that the polymer probe can be used for fluorescently navigated surgery. Moreover, the GE-11 targeted polymer probe exhibited the strongest signal in the periphery of the tumours, demonstrating the benefit of the probe for surgeons during the tumour resection. These EGFR-targeted fluorescent polymer probes should be further clinically investigated to accurately visualise the margins of the tumours intended for surgical resection. The future of modern oncology is undoubtedly associated with the use of targeted anti-tumour drugs and diagnostic probes, consequently fluorescently guided surgery and tumour-targeted therapy are potential future applications of the polymer-based multifunctional material platform described in this study.
Acknowledgments
This work was supported by the Ministry of Health of the Czech Republic (grant 16-28594A), by the Ministry of Industry and Trade of the Czech Republic (grant FV10370), by the Czech Science Foundation (projects 16-17207S and 17-13283S) and by the Ministry of Education, Youth and Sport of the Czech Republic via the National Sustainability Program I (project POLYMAT (LO1507).