Abstract
In this study, modified epoxy nanocomposite was produced by incorporating SiO2 nanoparticles of 15–30 nm in size, with different concentrations ranging from 1 to 20 wt%. The electrical properties of the epoxy nanocomposite were measured at room temperature in the frequency range of 10−2–107 Hz. To determine the impact of nanoparticles on the epoxy composition, scanning electron microscopy-energy dispersive x-ray spectroscopy (SEM-EDS), Fourier transform infrared spectra (FTIR) spectroscopy, and Raman spectroscopy were conducted. With an increase in filler (SiO2 nanoparticles) content, the electrical characteristics of the epoxy nanocomposite exhibited multiple changes. At low concentrations, all electrical properties experienced a notable increase. The epoxy with 15 wt% of SiO2 nanoparticles samples had a lower permittivity, loss number, conductivity, and capacitance than the unfilled epoxy. At medium concentrations (5 to 15 wt%), the formation of immobilized nanolayers has an impact on permittivity, loss number, conductivity, and capacitance, which have decreased; impedance and modulus increased. The initiation of contact between the nanofillers at a concentration of 20 wt% leads to the formation of continuous interfacial conductive pathways, resulting in a dramatic increase in the permittivity, conductivity, and capacitance of the composites, while concurrently reducing impedance.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanocomposite materials have garnered a lot of attention in the last 30 years. For instance, epoxy nanocomposite materials have special qualities that set them apart from metallic and ceramic materials, such as high break-down strength, mechanical flexibility, availability, degradability, low density, ease of processing, and low cost [1]. These qualities make them crucial parts of energy production and energy storage systems. Furthermore, functional polymer composites have several applications in energy resources, including power transmission, hybrid electric vehicles, high-power weaponry, radar, wind power generation, microelectronic devices [2], conversion of alternating current (AC) to direct current (DC); transmission engineering uses a high voltage direct current (HVDC) converter valve, and dielectric capacitors occupy more than half of the space in such systems [1]. Epoxy nanocomposites have also been used in the automotive sector since 1989 [2]. In field electron emission, the cathode is coated with a thin layer of epoxy nanocomposite to obtain a high emission current, increase the life of the emitter, and stabilize the current [3, 4].
Two major obstacles to creating a high-performance epoxy/silica nanoparticles (SNPs) composite are the homogeneity of the scattered silica nanoparticles in the matrix and their adherence with epoxy resin. To address these problems, numerous strategies have been developed to enhance the blending process and surface functionalization of silica nanoparticles [5, 6].
The nano-reinforced epoxy resin has garnered a lot of interest over the last 30 years because it can improve the electrical properties of epoxy [7]. Several methods have been suggested to illustrate the improved dielectric properties [8–12]. For example, George Tsagaropoulos and Adi Eisenberg explained the mechanism of formation of immobilized nanolayers between the epoxy chain and the nanoparticles at the glass transition temperature, which contributes to the constraining of the epoxy chain mobility [13]. Additionally, they demonstrated that the mobility of the polymer close to the immobilized layers is not greatly impacted when the filler content is lower than 5 wt%, due to the large average interparticle distance. Q Wang and G Chen studied the effects of the logarithmic Lichtenecker-Rothe mixing rule on the dielectric permittivity and breakdown behaviour of epoxy/nanocomposites, where both SiO2 and Al2O3 nanofillers were used in that work with minimum concentration 5 wt% [7, 13, 14]. Zhengdong Wang et. al studied the relationship between permittivity and conductivity of nano-epoxy composite with a high filler concentration of 40% They found that at the percolation threshold, the values of both permittivity and conductivity increase [15]. Liang Huang et al explain how the electrical pathways contribute to increasing the conductivity of the epoxy composite filled with Reduced graphene oxide (RGO)-encapsulated SiO2 hybrids (SiO2@RGO) at high concentrations of fillers up to 40% [16]. Another approach for enhancing permittivity by incorporating electrically conductive particles (Ag, Cu, Al, Zn, and carbon fillers) into a polymer, leading to a significantly high permittivity when the concentration of conductive fillers approaches the percolation threshold [17, 18]. In this work, the electrical properties (relative permittivity, loss factor, conductivity, impedance, capacitance, and modulus) of epoxy/SNPs filler samples have been described for three filler concentrations, low concentration (less than 5%), medium concentration (5% to 15%), and high concentration (20%). Each concentration value showed considerable changes in the electrical properties. In the low concentration, a minor rise has been observed in permitivity, conductivity, and capacitance and a drop in impedance and modulus. In contrast, the medium concentration shows a decline in permitivity, conductivity, and capacitance, as well as simultaneous increases in impedance and modulus. In case of high concentration, there was a significant increase observed in the values of permittivity, conductivity, and capacitance. which is accompanied by a decline in impedance and modulus. Importantly, the current study improved the composites' overall dielectric characteristics while also providing new insight into the design of epoxy nanocomposites by varying filler concentrations.
To make this work more traceable for the reader: the second section describes sample preparation. The material's characterization using SEM-EDS, FTIR, and Raman spectroscopy is also covered in the third section. The outcomes are detailed in section four. The conclusion is in section five. The publishers' outlook for future research is included in the last section,
2. Sample preparation
Epoxylite® E478 (E-478), purchased from Elantas (Wessel, Germany). High-purity SNPs (99.5%) with a particle size range of 3 to 15 nm was obtained from Sigma-Aldrich, USA. Different concentrations of SNPs were added to E-478, 1 wt%, 3 wt%, 5 wt%, 10 wt%, 15 wt%, and 20 wt%. Figure 1 shows the manufacturing steps for the epoxy nanocomposite samples. First, viscosity plays a key role in the dispersion of nanoparticles inside the epoxy, for that matter 6 g of epoxy resin and 2 ml of ethanol were placed in a container and mixed for 10 min to reduce the viscosity of the epoxy. After reducing the viscosity of the epoxy, the SNPs were added to the epoxy resin and ethanol mixture container and stirred manually for 10 min. The mixture container was put into an ultrasonic bath for 2 h to ensure a random distribution of SNPs. Then, the mixture was poured into a silicone mold that was resistant to elevated temperatures. The samples were placed in a furnace and cured in two stages: first, at 80 °C for 8 h to remove air bubbles from the mixture and to evaporate ethanol, then the mixture was annealed at 180 °C for one hour to harden the mixture. Finally, a micrometre was used to measure the sample thickness, which came out to be about 800 μm.
Figure 1. The production process of epoxy nanocomposite samples.
Download figure:
Standard image High-resolution image3. Characterization
3.1. Scanning electron microscopy—energy dispersive x-ray spectroscopy
Different characteristics analyses were conducted to confirm the successful preparation of the epoxy nanocomposite using SEM-EDS (MIRA-TESCAN, Czech Republic). SEM-EDS technique was utilized to observe the element distribution of the unfilled and nanocomposite samples. Figure 2 shows the elemental distribution of the unfilled epoxy, showcasing the individual representation silicon (Si). In the unfilled epoxy. Si concentration was found 1.68% of the epoxy components and it exhibited a near-uniform distribution across the entire sample surface. Figure 3 shows the distribution of silicon in the nanocomposite samples. It was also observed that there are large aggregations of silicon in the epoxy composites. These aggregations contribute to the formation of immobilized nanolayers between the silica and epoxy [7]. Table 1 shows weight percentages of silica in the unfilled and nanocomposite epoxy.
Figure 2. SEM-EDS mapping, including SEM image, of C, Si, O, Cl, and N for unfilled epoxy resin.
Download figure:
Standard image High-resolution imageFigure 3. EDS mapping, including SEM image, for the nanocomposite (A) epoxy/1 wt% SNPs, (B) epoxy/3 wt% SNPs, (C) epoxy/5 wt% SNPs, (D) epoxy/10 wt% SNPs, (E) epoxy/15 wt% SNPs, and (F) epoxy/20 wt% SNPs.
Download figure:
Standard image High-resolution imageTable 1. The weight percentages of silica in the epoxy resin nanocomposites were detected by EDS.
| Sample | Unfilled | Epoxy/1 wt% SNPs | Epoxy/3 wt. % SNPs | Epoxy/5 wt% SNPs | Epoxy/10 wt% SNPs | Epoxy/15 wt% SNPs | Epoxy/20 wt% SNPs |
|---|---|---|---|---|---|---|---|
| Si wt% | 1.68 | 2.82 | 7.43 | 11.86 | 14.67 | 18.24 | 21.33 |
3.2. Fourier transform infrared spectra
Transmittance spectra of the samples were acquired by vacuum Fourier transform infrared (FTIR) Vertex 70v (Bruker, USA). The silica nanoparticles impact on functional groups of the epoxy resin was investigated using an FTIR spectroscopy. Figure 4 shows the FTIR spectra of pure epoxy and epoxy/SNPs spectra, the assignments for their primary transmittance bands are listed in table 2, which display bands at 3065 cm−1 that are attributed to the C–H stretching mode of the epoxide group [19]. While those at 2957 and 2875 cm−1 are associated with the -CH2 and -CH3 stretching vibration modes of aromatic and aliphatic chains, respectively [5, 6, 9]. Some weakly intense bands at 1670 cm−1 show the presence of amine N-H functional groups of amines. The peaks at 1035 and 1570 cm−1 show the imide C–N bonds. Both N-H and C–N bonds are likely from the epoxy resin hardener [8, 20]. The medium to strong transmittance bands located at 1612 and 1507 cm−1 are related to the C=C and C-C stretching vibrations of the phenyl ring [8]. The characteristic transmittance band at 972 cm−1 allows for the identification of the epoxide groups contained in the resin [6, 9]. The sharp band at 830 cm−1 is attributed to the out-of-plane bending of the H-C= vibration occurring in maleimide rings [9]. The bands located at 570 cm−1 and 465 cm−1 were attributed to C–H out-of-plane transmittance of aromatic rings and Cl-C [5, 9], respectively. Finally, the weak peak of Si–OH and O–H bending peak at 3400 cm−1 [5, 6, 21]. As can be observed, the intensity nearly remained unchanged as the concentration of SNPs increased. This indicates that no new bonds were formed between the SNPs and epoxy resin. It is evident from the FTIR data that no bond appeared between silica and epoxy.
Figure 4. FTIR spectra of pure epoxy and epoxy/SNPs.
Download figure:
Standard image High-resolution imageTable 2. Band assignments of FTIR spectrum for Epoxylite® E478 (E-478).
| Wavenumber (cm−1) | Assignments |
|---|---|
| 465 | Cl-C stretching terminal |
| 570 | C–H out-of-plane transmittance of aromatic rings |
| 830 | C-H out-of-plane bending in maleimide rings |
| 972 | Epoxide groups |
| 1612 and 1507 | C=C and C-C stretching vibrations of the phenyl ring |
| 1035 and 1570 | Imide C–N bonds |
| 1670 | N–H functional groups of amine |
| 2957 and 2875 | –CH2 and –CH3 stretching modes of aromatic and aliphatic chains |
| 3065 | C–H stretching mode of the epoxide group |
| 3400 | Si-O and OH stretching |
3.3. Raman spectroscopy
The Raman microscope (Witec Alpha 300R- Oxford WiTec, Germany) was used to monitor the crosslinking response of epoxy resin and SNPs. To prevent any interference from any external light source, all spectrum was obtained without any ambient lighting. At room temperature, the Raman spectra of unfilled epoxy and six samples of epoxy/ SNPs with various concentrations of SNPs were recorded as shown in figure 5. It is evident from the Raman spectroscopy data that no bond appeared between silica and epoxy. Following that, it was determined which chemical groups were involved in the curing reaction.
Figure 5. Raman spectra of pure epoxy and epoxy/SNPs.
Download figure:
Standard image High-resolution imageFigure 5 illustrates that the epoxide ring vibration band is located at 1452 cm−1 [6, 22] , while the epoxide's vibrations are represented by Raman bands ranging from 1230 cm−1 to 1280 cm−1, these bands overlap other bands, -C-O-C- ether stretch at 1232 cm−1 and the aromatic C-H stretch at 3065 cm−1 [23, 24]. In addition to these bands, the symmetric CH2 stretching band at 2875 cm−1, associated with the formed link between the amine and epoxy, increases in intensity as the reaction proceeds [24], while the symmetric terminal epoxide =CH2 stretch at 3010 cm−1 disappears as the cure reaction proceeds [24, 25]. Raman peaks at wavelengths of 1112 cm−1 and 1608 cm−1 can be used to identify the resin backbone (phenyl band) vibrations [22]. Moreover, the C-H out-of-plane bending of the para-disubstituted phenyl group at 638 and 830 cm−1 [24, 25]. Raman shift values for epoxy composite samples are presented in table 3.
Table 3. Raman band assignment of epoxy resin.
| Raman shift (cm−1) | Assignment |
|---|---|
| 638 , 830, | C-H out-of-plane bending of the phenyl group |
| 1112 and 1608 | Phenyl group 
|
| Between 1230 and 1280 | Epoxide's vibration |
| 1232 | Epoxide vibration overlaps other bands, -C-O-C- ether stretch. |
| 1452 | Epoxide ring 
|
| 2875 | Symmetric CH2 stretching |
| 3010 | Symmetric terminal epoxide =CH2 stretch |
| 3065 | Epoxide vibration overlaps aromatic C–H stretch |
4. Results
4.1. Permittivity, tan(δ), and conductivity
Dielectric measurements were carried out on the Novocontrol Alpha-A analyser equipped with Quatro cryosystem by Novocontrol Technologies GmbH & Co. KG, Germany. The measurements were conducted across a wide frequency range spanning from 10−2 Hz–107 Hz. The duration of a single sweep did not exceed 15 ms and the measurement across the full frequency range with 63 measuring points takes about 35 min. The samples were investigated at room temperature. All samples and the electrode had a diameter of 20 mm and a thickness of 800 μm. A voltage of 1 VRMS was applied during the measurements.
The number of extant dipoles and their capacity to orient with an applied field at the measurement frequency determine the contribution of oriented dipoles to the measured dielectric permittivity. All dipoles orient in the low-frequency limit, and a high permittivity is seen. There is a decrease in permittivity at high frequencies where the dipoles are unable to obey the applied electric field. The dipolar relaxations are described by the Havriliak–Negami (HN) function, which is composed of the Cole-Cole and Cole-Davidson functions [20, 26], and it can be described as:
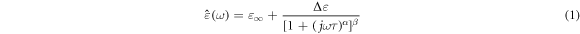
Where  the complex permittivity,
the complex permittivity, 
 is the permittivity at infinite frequency also called optical permittivity,
is the permittivity at infinite frequency also called optical permittivity,  is the static permittivity at zero frequency, α describes the flatness/width of the maximum, β expresses the skewness (asymmetry) of the complex dielectric permittivity, where α
is the static permittivity at zero frequency, α describes the flatness/width of the maximum, β expresses the skewness (asymmetry) of the complex dielectric permittivity, where α
 and 0 < β ≤ 1, these parameters show the changes in the relaxation time distribution. ω is the angular frequency and τ is the relaxation time. The complex permittivity can be decomposed into the real ε'(ω) and imaginary ε''(ω) parts as follows [26–28]:
and 0 < β ≤ 1, these parameters show the changes in the relaxation time distribution. ω is the angular frequency and τ is the relaxation time. The complex permittivity can be decomposed into the real ε'(ω) and imaginary ε''(ω) parts as follows [26–28]:
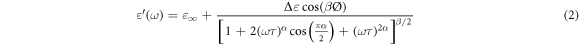
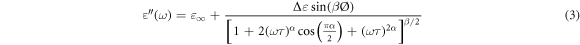
The energy dissipation of dielectric material during the application of an electric field is measured by the dielectric loss factor. Both the  and the tan(δ) are zero in the ideal condition [20]. In the case of the capacitor made by two plates separated by an epoxy nanocomposite, the dielectric loss factor is given by the ratio of the imaginary part and the real part of permittivity, and it can be written as [28] :
and the tan(δ) are zero in the ideal condition [20]. In the case of the capacitor made by two plates separated by an epoxy nanocomposite, the dielectric loss factor is given by the ratio of the imaginary part and the real part of permittivity, and it can be written as [28] :
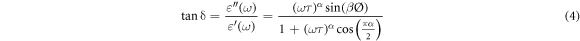
According to figure 6(a), the addition of 1 wt% and 3 wt% SNPs to epoxy led to the formation of easily polarizable bonds when subjected to an electric field. This contributes to increasing the relative permittivity of the epoxy/1 wt% and 3 wt% SNPs samples more than the relative permittivity of unfilled epoxy. However, the relative permittivity value started dropping when the SNPs concentration in the epoxy was raised to 5 wt% [5, 10]. This behaviour can be attributed to the interaction between the SNPs and polar groups within the epoxy, resulting in the formation of an immobilized nanolayer. However, at 5 wt% SNPs, the mobility of the polymer close to the immobilized nanolayers is not significantly affected due to the large average distance between the particles, but the relative permittivity value continues to decrease. In the case of 10 wt% and 15 wt% concentrations of SNPs, a noticeable reduction in the average interparticle distance. This decrease in interparticle distance hindered the mobility of epoxy chains between the silica nanoparticles (SNPs), consequently promoting the formation of an immobilized nanolayer. As a result, a significant decrease in relative permittivity was observed. In addition, the space charge polarization contributes to the reduction of the permittivity, where it arises due to the polarization between the base resin and the nanoparticles resulting from the accumulation of space charges within the resin system [5, 27, 29]. But since interfacial polarization occurs in the lower frequency bands up to 103 Hz, it can be ignored.
Figure 6. Variations of 

 with respect to frequency, and Cole-Cole plot.
with respect to frequency, and Cole-Cole plot.
Download figure:
Standard image High-resolution imageA distinct rise in dielectric permittivity was observed at an SNPs concentration of 20 wt%. This sudden increase in active permittivity can be explained by the logarithmic Lichtenecker-Rothe mixing rule': where the permittivity of the resulting nanocomposites is more significantly influenced by the permittivity of the filler as the loading concentration increases. The logarithmic Lichtenecker-Rothe mixing rule can be written as [5, 27]
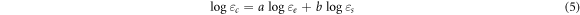
where,  is the relative permittivity of the epoxy nanocomposite,
is the relative permittivity of the epoxy nanocomposite,  is the permittivity of epoxy,
is the permittivity of epoxy,  is the permittivity of silica, while a and b are the concentrations of epoxy and silica, respectively. Since the permittivity of silica is higher than that of the epoxy resin [10], this may be one of the factors that led to high permittivity at higher concentration loading. Furthermore, percolation theory, which is considered a typical theory in polymer nanocomposites, can be employed to explain this phenomenon [16, 30, 31]. The dielectric permittivity of epoxy nanocomposites near the percolation threshold can be described by equation (6) as follows [16, 32]:
is the permittivity of silica, while a and b are the concentrations of epoxy and silica, respectively. Since the permittivity of silica is higher than that of the epoxy resin [10], this may be one of the factors that led to high permittivity at higher concentration loading. Furthermore, percolation theory, which is considered a typical theory in polymer nanocomposites, can be employed to explain this phenomenon [16, 30, 31]. The dielectric permittivity of epoxy nanocomposites near the percolation threshold can be described by equation (6) as follows [16, 32]:
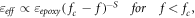
where  is the effective permittivity,
is the effective permittivity,  dielectric permittivity of the matrix, f is the volume fraction of the filler,
dielectric permittivity of the matrix, f is the volume fraction of the filler,  is the percolation threshold and s is the critical exponent. As expected, the dielectric properties of the epoxy nanocomposite show a typical percolation transition behaviour as the mass fraction of SNPs in the epoxy matrix increases. The approximate percolation threshold for the epoxy/SNPs nanocomposites is near 20 wt%.
is the percolation threshold and s is the critical exponent. As expected, the dielectric properties of the epoxy nanocomposite show a typical percolation transition behaviour as the mass fraction of SNPs in the epoxy matrix increases. The approximate percolation threshold for the epoxy/SNPs nanocomposites is near 20 wt%.
Figure 6(b) depicts the imaginary part of the relative permittivity as it appears in composite samples. At frequencies lower than 10 Hz, the increase in the imaginary part of the relative permittivity is due to interfacial polarization. It turned out that the addition of SNPs up to 5 wt% increased the number of electric dipoles, leading to an increase in interfacial polarization. Consequently, this led to an increase in the imaginary part of the relative permittivity. The formation of immobilized nanolayers was observed to reduce the value of the imaginary relative permittivity, for samples epoxy/10 wt% of SNPs and epoxy/15 wt% of SNPs, due to the obstruction of the immobilized nanolayers to the movement of space charges. However, increasing the concentration of SNPs to 20 wt% led to a rise in the  value which can be explained by the mixing rule. At high frequencies, the peaks observed at 106 Hz in figure 6(b) are associated with the β-relaxation, which is indicative of the motion of dipolar species generated during the curing process. It was observed that the position of relaxation peaks did not change with the increase of the filler concentration.
value which can be explained by the mixing rule. At high frequencies, the peaks observed at 106 Hz in figure 6(b) are associated with the β-relaxation, which is indicative of the motion of dipolar species generated during the curing process. It was observed that the position of relaxation peaks did not change with the increase of the filler concentration.
Additionally, figure 6(c) shows the variation in loss factor (tan δ) value for both epoxy nanocomposites and unfilled epoxy. The tan δ value of epoxy/SNPs and unfilled epoxy sample dropped at higher frequency ranges. The unfilled epoxy and epoxy/SNPs exhibited a minimum value of tan δ at approximately 100 Hz. After this point, the tan δ values began to rise until they reached their maximum values around 106 Hz. The tan δ value of unfilled epoxy is lower than that of epoxy/SNPs samples in the lower frequency range (less than 100 Hz) except for epoxy/15 wt%. At 20 wt% of SNPs concentration, owing to the formation of electrical paths in the epoxy matrix, the dielectric loss also increases near the percolation threshold, whereas the β-relaxation property of net epoxy resin predominates in the fluctuation in the range of 106 Hz [16].
Figure 6(d) shows the relationship between ε'(ω) and ε''(ω) using Cole—Cole diagram for all epoxy nanocomposites samples. The results indicate depressed semicircles with dispersed relaxation times and non-Debye behaviour. The complex permittivity plot ε'(ω) versus ε''(ω) is known to be significantly distorted at its low-frequency region for materials with high DC conductivity [33].
The semicircle is attributed to the bulk relaxation process, and it indicates the presence of a capacitive component. The semicircle is followed by a linear increase for all epoxy nanocomposite samples. Moreover, the line is ascribed to electrode polarization on the interface between the sample and the electrodes where the conductivity increases for the material [34]. Table 4 shows the frequency value at which epoxy nanocomposites are converted into a conductor.
Table 4. The frequency value at which the epoxy nanocomposites are converted into a conductor.
| Sample | Unfilled epoxy | Epoxy/ 1% SNPs | Epoxy/ 3% SNPs | Epoxy/ 5% SNPs | Epoxy/ 10% SNPs | Epoxy/ 15% SNPs | Epoxy/ 20% SNPs |
|---|---|---|---|---|---|---|---|
| Frequency (kHz) | 0.81 | 1.6 | 1.6 | 1.6 | 1.13 | 1.13 | 0.58 |
4.2. Electrical conductivity
Due to constraints on the generation of mobile charge and the movement of charge carriers in polymer dielectrics, which are caused by the presence of SNPs inside epoxy, the electric conductivity will decrease, especially at lower frequencies where the conductivity will be more crucial. Thus, the electrical conductivity of SNPs is a crucial factor in changing the value of  in the low-frequency range. The real part of the conductivity
in the low-frequency range. The real part of the conductivity  can be expressed as [35, 36]:
can be expressed as [35, 36]:
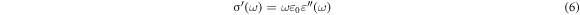
The epoxy/1 wt% SNPs sample in figure 7 shows the highest conductivity values, due to the increased space charges within the sample. The value of conductivity begins to decrease gradually, starting from a concentration of 3 wt% due to the restriction of the mobility of space charges. At a concentration of 5 wt% SNPs, immobilizer nanolayers begin to form, consensually, decreasing epoxy nanocomposite conductivity. Also, at a concentration of 15 wt% SNPs, the conductivity of the epoxy nanocomposite is less than the conductivity of the unfilled epoxy due to the significant increase in the formation of immobilized nanolayers.
Figure 7. Variations of conductivity with respect to frequency.
Download figure:
Standard image High-resolution imageThe conductivity of the epoxy/20 wt% SNPs sample suddenly increased to a value higher than that of the unfilled epoxy, due to the formation of the conductive pathway. At the 20 wt%, which represents the concentration threshold, the fillers were unable to effectively establish full contact with each other, resulting in a limited increase in conductivity compared to the unfilled epoxy. Due to their insulating nature, the conductivity curves show a strong dependence on the frequency.
It is expected if the filler concentration rises to 30 wt% or more, the conductivity further increases, and an insulator–semiconductor transition will be observed. This is caused by the formation of effective physical electrical pathways in the composites. At this filler loading content, the composites exhibit a conducting feature that is nearly frequency independent. This conclusion is supported by the literature [15, 16].
Based on figure 7, all epoxy nanocomposite results exhibit two threshold frequencies, f1 and f2, which divide the entire variance into three regions. The frequency response of conductivity can be interpreted in terms of the jump relaxation model [37–40]. This model explains the mechanism of ion transport in a dielectric material, where the frequency dependence is related to relaxation processes of the jumping ions environment [37, 38]. At low-frequency region, when f < f1, the conductivity is almost frequency-independent ( [39]. And the
[39]. And the  is a few orders of magnitude less than the AC conductivity (
is a few orders of magnitude less than the AC conductivity ( This is because the
This is because the  is determined by the most complex transition in complete percolation channels between the electrodes. Whereas
is determined by the most complex transition in complete percolation channels between the electrodes. Whereas  is governed by the easiest local movement of the charges [40].
is governed by the easiest local movement of the charges [40].
At moderate frequencies region, where  the conductivity rises linearly with the frequency. This shows that the translational hopping motion corresponds to the conduction mechanism in this area [39, 40]. At high frequencies region, where
the conductivity rises linearly with the frequency. This shows that the translational hopping motion corresponds to the conduction mechanism in this area [39, 40]. At high frequencies region, where  the conductivity increases linearly with the frequency. This demonstrates that the well-localized hopping and/or reorientation motion corresponds to the conduction mechanism in this range of frequencies [39, 40].
the conductivity increases linearly with the frequency. This demonstrates that the well-localized hopping and/or reorientation motion corresponds to the conduction mechanism in this range of frequencies [39, 40].
4.3. Impedance
Impedance is the resistance to alternating current (AC) flow in a complex system. A passive complex electrical system includes two elements, the energy dissipator (resistor) and energy storage (capacitor). In this work resistor and capacitance were connected in series. Impedance is a complex quantity because the current has a different phase than the applied voltage and it can be represented by the following formula [35]:
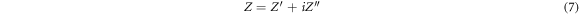
where  is the real part impedance and
is the real part impedance and  is the imaginary part impedance and both can be written as [41]:
is the imaginary part impedance and both can be written as [41]:
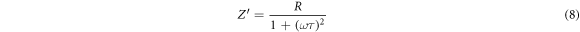
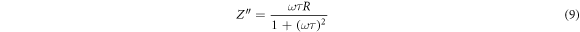
where τ is the relaxation time (τ = RC), and ω is the angular frequency. Figure 8 exhibits frequency-dependent plots of log |Z''| as a function of frequency. From figure 8(a), it can be depicted that the value of Z' declines with the increase in frequency. The impact of free charges on impedance becomes noticeable below 0.1 Hz, as it appeared in figure 8(a). It can be depicted that the value of Z' declines continuously with the decrease in frequency. Moreover, the impedance value decreased to its lowest level when the filler concentration was 1 wt% SNPs even lower than the impedance value of the unfilled epoxy. At 3 wt% and 5 wt% of SNPs concentration, the impedance gradually starts to rise, but it continues to be lower than that of the unfilled epoxy. So, it can be concluded that when the concentration of silica filler is less than 5 wt%, there is an increase in space charges as explained earlier, which decreases the impedance value, signifying an increase in conductivity [42]. Whereas, when increasing the filler concentration between 10 wt% and 15 wt%, an increase in the resistivity value due to the formation of immobilized nanolayers that limit charge polarization, as detailed previously. The effects of the mixing rule and percolation threshold become evident in the case of a 20 wt% concentration, manifesting as an increase in impedance value. Nevertheless, a concurrent rise in conductivity is observed. These findings in the present section are consistent with the results presented in section 3.1 pertaining to permittivity and conductivity.
Figure 8. Variation of Z ' with log |Z''|' as a function of frequency.
Download figure:
Standard image High-resolution imageFigure 8(b) shows the frequency dependence of Z' and reveals a transition between capacitive and ohmic behaviour. A linear increase at high frequency is followed by a small quasi-constant plateau at low frequency. Additionally, as filler concentration rises, the plateau's shift towards the high-frequency band suggests modifications to the electrical characteristics and ionic behaviour [42]. Generally, this result is matched with the results for the imaginary part of permittivity obtained in section 3.1.
4.4. Capacitance
When examining the capacitance response to frequency variations it was as depicted in figure 9. Based on the figure, capacitance depends on variations in the epoxy nanocomposite's permittivity value. Due to the enhanced permittivity (see figure 6(a)), the capacitance values at 1 wt% and 3 wt% of SNPs increased and are greater than those of the unfilled epoxy. However, at concentrations of 3 wt%, 5 wt% and 10 wt% of SNPs the capacitance value continued to fall, and at 15 wt% of SNPs, it nearly reached the capacitance value of unfilled epoxy. The high value of capacity at low frequencies may be attributed to space charge polarization.
Figure 9. Variation of capacitance as a function of frequency.
Download figure:
Standard image High-resolution imageIt is important to note that the capacity dramatically increased when the filler concentration reached 20%, which is a result of the rise in permittivity at this concentration, despite the increased conductivity. That was covered in section 3.1.
The dielectric constant of a substance or material signifies its ability to store electrical energy. It quantifies how much electric flux a substance can store or concentrate. Mathematically, the dielectric constant is defined as the ratio of a material's capacitance  to the capacitance of free space
to the capacitance of free space  as in equation (10). Additionally, it serves as the electrical equivalent of relative magnetic permeability [43].
as in equation (10). Additionally, it serves as the electrical equivalent of relative magnetic permeability [43].
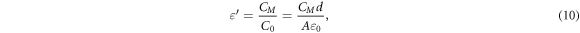
where  the permittivity of the free space, d is the distance between the electrode, and A is the area of the electrode. The dielectric constant values are calculated by putting the values
the permittivity of the free space, d is the distance between the electrode, and A is the area of the electrode. The dielectric constant values are calculated by putting the values  = 8.854 × 10−12 CV−1 m−1, A = 12.56 cm2 and d = 800 μm. Using equation (10), the values of the dielectric constant are determined. Table 5 shows the capacitance and dielectric constant values for all samples at room temperatures at frequency of 10−2 Hz.
= 8.854 × 10−12 CV−1 m−1, A = 12.56 cm2 and d = 800 μm. Using equation (10), the values of the dielectric constant are determined. Table 5 shows the capacitance and dielectric constant values for all samples at room temperatures at frequency of 10−2 Hz.
Table 5. Shows the capacitance and dielectric constant values for all samples at room temperatures, at a frequency of 10−2 Hz.
| Sample | Unfilled | Epoxy/ 1 wt% SNPs | Epoxy/ 3 wt% SNPs | Epoxy/ 5 wt% SNPs | Epoxy/ 10 wt% SNPs | Epoxy/ 15 wt% SNPs | Epoxy / 20 wt% SNPs |
|---|---|---|---|---|---|---|---|
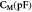
| 0.13 | 0.19 | 0.22 | 0.2 | 0.15 | 0.12 | 0.27 |

| 4.5 | 6.7 | 7.9 | 7.3 | 5.9 | 4.4 | 9.7 |
4.5. Electrical modulus
In the frequency domain, the complex electrical modulus and complex electrical permittivity are related by the following [1]:
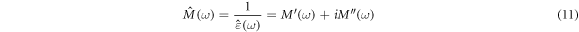
The real  and imaginary
and imaginary  part of the complex electrical modulus is calculated using the following relation [41, 43]:
part of the complex electrical modulus is calculated using the following relation [41, 43]:
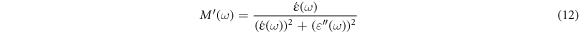
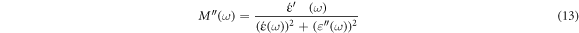
Figure 10. Depict the frequency-dependent electric modulus M' and M'', respectively, of both unfilled epoxy and epoxy/SNPs composites as a function of frequency.
Figure 10. Variation of M' and M'' as a function of frequency.
Download figure:
Standard image High-resolution imageFigure 10(a) illustrates the real part of the modulus. The epoxy with a 1% filler content exhibits a lower modulus value compared to the unfilled epoxy. The minimum modulus value is observed at a filler content of 3%. Thereafter, the modulus value exhibits a steady increase as the filler concentration escalates to 5%, culminating in its peak value at a filler concentration of 20%.
The imaginary part of the electric modulus spectra is shown in figure 10(b). Very clear relaxation peaks are seen in the M'' spectra for samples at 106 Hz. Since ions are largely immobilized due to constrained chain segment motions at room temperature, ion polarization can be excluded. In addition, considering the fact that the relaxation also occurs in pure epoxy resin, Maxwell–Wagner interfacial polarization due to charge accumulation at heterogeneous contacts can be ruled out. Therefore, the relaxation process should be ascribed to the orientation of small polar groups such as those in side chains or side groups. The polar entities can be residual amines -NH2- and -NH-, hydroxyl, epoxide rings, and other possible dipolar species [44].
The significant increase in the M in epoxy/SNPs at 106 Hz is attributed to the common effects of hopping conductance loss, dipole polarization loss and microscopic interface polarization loss [44]. This can be seen in samples of epoxy/10 wt% of SNPs and epoxy/15 wt% of SNPs due to the formation of nucleated immobilized nanolayers. In other words, the material becomes more insulating. Finally, a noteworthy reduction in M'', static dielectric interfacial polarizability, static dielectric dipolar polarizability, and hopping conductivity is observed upon the addition of both low concentration (epoxy/1 wt% and epoxy/3 wt% SNPs) and high concentration (epoxy/20 wt% SNPs) of SNPs. This observation can be attributed to the presence of space charges and the formation of conductive pathways, ultimately resulting in increased material conductivity.
5. Conclusions
Dielectric spectroscopy has been used to examine the relative permittivity and loss number (ε''), loss factor (tan δ), conductivity, impedance, capacitance, and modulus of unfilled epoxy and epoxy/SiO2 nanoparticles loaded with low concentration (less than 5 wt%), medium concentration (10 wt% and 15 wt%) high concentration (20 wt%). It was shown from the results of FTIR and Raman spectroscopy that the silica nanoparticle did not create many bonds with the epoxy. However, three key findings can be distilled from this work:
- i-Compared to the unfilled epoxy, the low filler concentration increases relative permittivity, conductivity, and capacitance values, while decreasing impedance and modulus values. This effect can be attributed to the increase in space charge.
- ii-In the filler concentration range of 5 wt% to 15 wt% of SNPs, permittivity, conductivity, and capacitance values decreased. However, both the modulus and impedance increased due to the formation of immobilized layers, which significantly restrict the polarization of charges.
- iii-At a filler concentration of 20 wt%, the filler's permittivity starts to influence the epoxy compound's overall permittivity, which causes an increase in all relative permittivity, capacitance, and modulus, and this is based on the mixing rule. An increase in conductivity was recorded due to the formation of conductive pathways when the percolation threshold was reached, and this was matched by a significant decrease in impedance compared to unfilled epoxy.
6. Future research
The outcomes of this study provide a solid foundation for future research initiatives. These forthcoming investigations will encompass an in-depth exploration of temperature effects on the electrical properties of epoxy nanocomposites. Furthermore, we plan to extend our research scope to encompass other nanoparticles, including aluminium oxide and magnesium titanium, and assess their impact on the electrical characteristics of epoxy nanocomposites. In parallel, our research will delve into the influence of high-voltage conditions on epoxy nanocomposites. As part of an advanced phase, our research will also scrutinize the influence of epoxy nanocomposites on electron emission.
Acknowledgments
Czech Nano Lab project LM2023051 funded by MEYS CR is gratefully acknowledged for the financial support of the measurements/sample fabrication at CEITEC Nano Research Infrastructure. We also acknowledge the Czech Academy of Sciences (RVO: 68081731) for providing the etching equipment and laboratory infrastructure.
Data availability statement
The study is part of ongoing research. The data that support the findings of this study are available upon reasonable request from the authors.












