Abstract
The inactivation of Bacillus subtilis (ATCC 6633) spores deposited on a filter membrane was studied by using low-temperature plasma produced via surface dielectric barrier discharge. Spore samples were carefully prepared to avoid the formation of cell aggregates, and their inactivation was induced by multiple surface streamer discharge driven in a coplanar dielectric barrier discharge electrode geometry by an amplitude-modulated AC high voltage waveforms in humid air at atmospheric pressure. At a discharge duty cycle of 0.4, the surface dielectric barrier discharge is characterised by an average total power of 1.7 W (power density 1.5 W cm−2 and energy density ∼0.3 Wh l−1) and a low gas temperature of the plasma filaments of about 320 K. The spores were exposed by placing a sample holder at a fixed distance of 3 mm from the electrode surface covered by plasma filaments. Particular attention was paid to identifying sporicidal agents employed in the process of inactivation. Since treated samples did not come into direct contact with the streamer filaments and excessive heating was excluded thanks to the low energy density, our results indicate that the spores were inactivated mainly by reactive oxygen and nitrogen species such as O3, H2O2 and NO2–. Discharge-induced damage of the spore structure was evidenced via the detection of dipicolinic acid and leaking of intracellular components. We therefore conclude that B. subtilis spores were inactivated chemically, probably due to failure of the coat structure or membrane of the spore.
Export citation and abstract BibTeX RIS
1. Introduction
Dormant bacterial spores are much more resilient to numerous methods of eradication than their vegetative equivalents. The high level of resistance to decontamination techniques and possible pathogenicity of a number of bacterial spores require new methods that might be able to kill them. Interest in the application of low-temperature plasmas for decontamination is increasing, and the results are sufficiently promising to study this way of decontamination in more detail.
Various low-temperature plasma sources (e.g. dielectric barrier discharges (DBD), plasma jets and corona discharges) and plasma-assisted techniques have been intensively studied for a wide range of applications, including bio-applications [1–5]. The use of DBDs seems to be particularly suitable for bio-decontamination, since a dielectric material separating conductive electrodes prevents spark formation and consequently excessive heat loads on exposed objects. DBDs are efficient in producing reactive species that may play a significant role in bacterial decontamination. The cost of plasma-induced decontamination depends on the operating conditions of the discharge apparatus (i.e. the electrode geometry, discharge power, gas mixture) since these determine the concentrations of reactive species (electrons, ions, radicals) and consequently the various chemical and physical processes leading to the killing of bacteria [6].
Various microorganisms were found to be susceptible to plasma treatment, including bacteria, spores, yeast and viruses. Generally, spores appear to be very resistant bacterial organisms; however, low-temperature plasmas seem to be efficient decontamination agents for spores [7–16]. The structure and chemical composition of the spores appear to be important factors in their resistance. Spores have various mechanisms for resisting to conventional decontamination techniques, many of which rely on chemical composition. Several resistive mechanisms have been developed in spore cells, including a large production of dipicolinic acid (DPA, pyridine-2,6-dicarboxylic acid), which is synthesised during sporulation and it is released in the early stages of germination or during heating. Moreover, the water content in the spore coat is remarkably low. The structure of the DPA in the core may play a role in the lower water content and resistance to UV–induced DNA damage [17–20]. In addition, small acid-soluble proteins (SASP) play a significant role in preventing DNA damage, and directly protect DNA backbone by binding to the DNA structure and changing the conformation [17, 19, 21–23]. Additionally, a significant resistance process in case where DNA is damaged involves excision repair during spore germination [23, 24]. Although there are numerous strategies for the survival of spores, several processes are able to damage them; this includes exposure to strong acids, which causes a rupturing of the spore permeability barrier, inducing release of DPA [25].
As in any other plasma-chemical application, the efficacy of the treatment depends on various discharge operating conditions, such as discharge electrode geometry, mean dissipated power and feed gas flow and composition, all of which influence the chemical and physical processes of the discharge. In addition to the biomedical applications, attention needs to be paid to the various methods by which biological targets are prepared and exposed to the plasma, as these can have a profound influence on the reproducibility of the results and the efficacy of treatment. Due to the enhanced resistance of spores in the process of decontamination, bacterial samples must be prepared with great care. Recently, Shama et al proposed a universal protocol for preparing samples of B. subtilis spores as a reference biological agent for comparing various plasma-assisted inactivation techniques across laboratories [26, 27]. The most important advantage of this protocol is that microorganisms are prepared that are always in the same growth phase. It has been shown that a minor change in sporulation temperature affects the resistivity of spores to decontamination, including plasma-assisted methods [28, 29]. Another improvement is that microorganisms are homogeneously distributed on the treated surface. It has also been demonstrated that suspended particles or microorganisms in an evaporating droplet migrate to the edge, creating the ring-like concentrated aggregates [30, 31].
In this work, we focus on the inactivation of Bacillus subtilis (ATCC 6633) spores, prepared by a universal reference protocol, with particular emphasis on the mechanisms by which the plasma interacts with spores. The most important aspect of the protocol is the use of a filtration technique to deposit cells in a single monolayer, thus avoiding the formation of cell aggregates which might, in turn, affect the inactivation kinetics [32]. This paper is organised as follows. The next section describes the novel multiple filamentary coplanar surface dielectric barrier discharge (CSDBD) reactor used to treat bacterial samples, the opto-electrical diagnostics used to characterise the discharge, the protocol used to prepare/analyse bacterial samples and the techniques/assays used to quantify various products. The following sections report on the detection of reactive species that contribute to spore inactivation. Finally, we provide experimental evidence of spore inactivation and discuss the crucial role of reactive species, supported by the observation of structural changes through the release of DPA and other intracellular components.
2. Experimental setup, materials and methods
2.1. CSDBD plasma source and related opto-electrical diagnostics
The geometry of the CSDBD electrode allows for the production of thin plasma filaments distributed on the surface of a dielectric material [33, 34]. Figure 1 shows a simplified sketch of the CSDBD reactor used in this study. The reactor used to test the interaction between a multiple surface streamer micro-discharge and biologically contaminated samples consists of a concentric CSDBD electrode system placed in a compact 3D-printed flow-through chamber, designed in our lab and equipped with gas feed input/output ports, a high-voltage interface and a sample holder. The modular concept of the reactor allows for the quick insertion of treated samples and very straightforward replacement of the discharge electrode(s) to use alternative discharge and/or treatment geometries [35]. Multiple filaments were produced on the surface of the CSDBD electrode, a disc of diameter 25.4 mm thickness 5 mm, made from MACOR® machinable glass-ceramic material and sealed in a polyamide holder. Multiple-streamer micro-discharge was initiated on the ceramic surface. The driving electric field was induced by high-voltage (HV) waveforms imposed between two concentric ring-shaped silver electrodes, embedded approximately 0.4 mm below the MACOR® surface with a minimum distance of 1 mm between them. The reactor was powered by an amplitude-modulated AC HV power supply composed of a TG1010A Function Generator (TTi), a Powertron Model 250A RF amplifier and a HV step-up transformer, as in a previous study [36]. The micro-discharges were produced by a burst of four sine-waves (uHV = 22 kV peak-to-peak, fAC = 5 kHz) applied at a fixed repetition frequency of fM = 500 Hz, with a resulting duty cycle of 0.4.
Figure 1. Experimental setup for testing the inactivation of B. subtilis spores: (a) simplified sketch of the compact reactor chamber; (b) Macor®-based CSDBD axially symmetric electrode system with embedded circular electrodes; and (c) snapshot of typical filamentary coplanar micro-discharges produced during one AC cycle in humid air at atmospheric pressure.
Download figure:
Standard image High-resolution imageA Tektronix oscilloscope (DPO5204, 2 GHz, 10 GS s−1) was used to record the discharge characteristics, i.e. the HV, current and plasma-induced emission (PIE) waveforms. The discharge HV waveforms were sampled by a Tektronix P6015 HV probe. The current pulses produced by individual micro-discharges were monitored via the voltage drop across a non-inductive shunt resistor inserted between the grounded electrode and the grounding lead, and were measured by a Tektronix TPP1000 HV probe. The transferred charge was determined by inserting a non-inductive measuring capacitor (C = 0.47 μF) into the grounding lead and analysing the charge-voltage characteristic (Lissajous figures). The reactor was fed with humid synthetic air through a Bronkhorst HI-TEC model mass-flow controller (flow rate Q = 0.1 l min−1). The vapour density was about 12.1 g m−3 (relative humidity of 70% measured at 20 °C). The distance between the surface of the CSDBD electrode and the exposed sample was adjustable, and was fixed at 3 mm in all of our experiments.
Two frontal windows allowed for direct visual control and optical emission diagnostics of the discharge area. A fast Hamamatsu R2949 photomultiplier (PMT) and Andor iStar ICCD DH740i-18U-03 camera were used to record the optical emission waveforms and the spectra collected from the whole surface of the CSDBD electrode. The ICCD was used to acquire the time-averaged emission spectra through the iHR-320 spectrometer.
2.2. Preparation and treatment of B. subtilis spore samples
The preparation of B. subtilis spore stock and deposition on the filters was carried out according to the protocol detailed by Dolezalova et al and Shaw et al [26, 27]. The advantage of this protocol lies in setting up of a universal biological reference protocol for the study and comparison of plasma treatments across laboratories. This protocol ensures (i) the production of microorganisms in a specific growth phase, and (ii) the standardised preparation of bacterial samples made of spores distributed in single monolayers. Both of these appear to be critical issues that can impede the direct comparison of inactivation results achieved in different labs. Spore resistance strongly depends on the conditions leading to sporulation, and the use of a 30 min thermal shock at 70 °C to initiate sporulation is therefore recommended.
The protocol also includes a novel technique for the deposition of spore samples on the filter membrane. Until now, pipetting a bacterial solution onto a substrate has been one of the most commonly used techniques in the study of the bactericidal properties of various plasmas. Unfortunately, the microbial distribution produced by pipetting a bacterial suspension as a droplet is neither homogeneous nor reproducible. Furthermore, heterogeneously distributed bacteria can migrate, creating clusters and consequently forming cell refuges that are not exposed to plasma. Pipetting is therefore unsuitable for plasma-assisted sporicidal testing.
In this procedure, vegetative cells of B. subtilis (ATCC 6633, Czech Collection of Microorganisms, Brno, Czech Republic) were pipetted onto Petri dishes containing sporulation agar (Himedia, Mumbai, India). Petri dishes were incubated at 30 °C for three weeks. Sterile physiological solution (5 ml) was added to the Petri dishes in order to harvest the spores. The surface was carefully scraped with a sterile inoculation loop to separate the spores from the surface of the agar, and the suspension was centrifuged in order remove agar debris. The supernatant was poured away and 10 ml of fresh sterile physiological solution was added, followed by vigorous shaking in order to re-suspend the spore pellets. This process was then repeated twice, in order to remove the majority of the remaining debris. The resulting spore suspension, contained in a sterile glass bottle, was transferred to a water bath at 70 °C for 30 min. After the sporulation process, the suspension was stored at 4 °C until handling, without loss of viability.
Before any individual plasma-assisted treatment of spores was carried out, 1.2 ml of the initial bacterial suspension (0.5 × 108 CFU ml−1) was filtered through a sterile polycarbonate filter membrane (with diameter and porosity of 13 mm and 0.4 μm, respectively; Whatman, GE Healthcare Life Sciences, Tonglu, China) in order to deposit a single monolayer of spores. Filtration was carried out through a rubber bung on a top of a Büchner flask using a vacuum pump. Once the spores were deposited on the filter, the filter was removed from the filter holder using tweezers, placed on the autoclaved glass slide and inserted in the CSDBD reactor on the sample holder at a fixed distance from the surface of the CSDBD electrode.
After treatment in the reactor, both exposed (discharge on) and control (discharge off) spore samples were recovered by transferring the filter into a sterile bottle containing physiological solution and six ballotini beads; the tube was then placed into an Eppendorf shaker for 45 s. The obtained spore suspension was diluted to the desired concentration and 100 μl was spread onto a tryptone soya agar plate with a disposable spreader. The Petri dishes containing B. subtilis were incubated at 30 °C for 24 h and the colony forming units (CFU ml−1) were counted.
2.3. Analysis of the discharge products
As the principal gaseous discharge products, ozone and NxOy species were sampled at the output port of the CSDBD reactor. A non-dispersive UV absorption ozone monitor API 450 and chemiluminiscence NOx analyser model 200EM (both Teledyne Instruments) were used, with a minimum airflow rate of 0.5 l min−1, to quantify the discharge products in both dry and humid air.
Chemical measurements of H2O2, NO2− were performed using the sample holder, which was designed to contain 2.5 ml volume of liquid. The water surface was placed bellow the discharge at a distance of 3 mm from the electrode, maintaining similar conditions as in the case of the treatment of the spores.
The concentration of hydrogen peroxide was measured using a titanyl sulfate reagent solution, which reacts selectively with H2O2 [37]. Sodium azide was added prior to the mixing of the sample with the titanyl sulfate reagent [38]. The absorbance at 407 nm of the yellow complex formed with H2O2 was recorded using a microplate reader (Varioskan® Flash).
The concentration of nitrites was measured using Griess reagent. The Griess reaction is based on the diazotisation reaction of sulphanilamide and N-1-napthylelthylenediamine dihydrochloride under acidic conditions [39, 40]. The absorbance at 540 nm of the pink complex formed with NO2– was recorded using a microplate reader (Varioskan® Flash).
2.4. DPA and other intracellular components assays
A DPA assay was carried out following Warth [41]. In brief, the spores from the filter were suspended in a 5 mM Ca2+, Tris buffer at pH 7.6 and centrifuged. The DPA concentration was recorded based on the difference in the two peaks at 276 nm (maximum) and 280 nm (minimum) using a microplate reader (Varioskan® Flash). The concentration of DPA was calculated based on the prepared standard curve in the range from 0 to 10 μg ml−1 of purified DPA (Sigma, St. Luis, USA)
The leaking of intracellular components (DNA, RNA, and proteins) can be revealed by UV absorption. We selected a wavelength of 260 nm, corresponding to the peak absorbance of DNA. For these tests, plasma treated spores were suspended in 250 μl of physiological solution and centrifuged (3 min at 12 000 rpm) to remove any cells or cellular debris. The absorbance of supernatant was then recorded by a microplate reader (Varioskan® Flash) at 260 nm (i.e. the wavelength of maximum absorption for DNA) [42–44]. Assuming the presence of the DNA in the liquid, its concentration was estimated assuming that an absorbance of one is equivalent to 50 μg ml−1 of pure DNA.
3. Results and discussion
3.1. Discharge characteristics
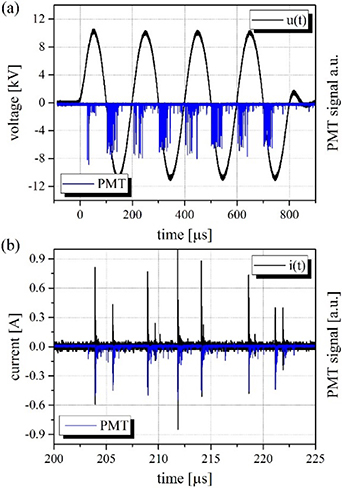
The CSDBD driven in the amplitude-modulated regime at a fixed AC HV amplitude of 22 kV (peak-to-peak) and a duty cycle of 0.4 produces between seven and nine strong micro-discharge filaments during both the positive and negative half period of any AC cycle, as evidenced by the snapshot in figure 1(c). Figure 2 shows the typical voltage-current-photomultiplier waveforms that can be observed during a single burst. All PMT waveforms were acquired with no spectral filter, and the PMT signal is therefore given by the total UV–vis-NIR discharge emission modulated by the efficiency of the PMT's photocathode. In addition, figure 2(b) clearly shows the synchronous occurrence of the current and PMT pulses.
Figure 2. Opto-electrical characteristics of the axially symmetric CSDBD produced in humid air: (a) burst of four sinus driving HV and PIE waveforms and (b) current and PIE pulses produced by individual CSDBD micro-discharges during second positive AC half-cycle. Discharge parameters were as follows: uAC = 22 kV peak-to-peak, fAC = 5 kHz, fM = 500 Hz, Q = 0.1 l min−1. Acquired as a single trigger event.
Download figure:
Standard image High-resolution imageThe production of micro-discharges occurs with a certain distribution [33] along the rising slope of a positive half-period with a typical pulse-to-pulse separation of few microseconds. A similar trend can be observed during any negative half-cycle. Figures 3(a) and (b) show the characteristic distributions of micro-discharges during both the positive (a) and negative (b) half-cycles captured by the PMT waveforms for a given HV amplitude of 22 kV (peak-to-peak), acquired as an average of 1024 samples (DPO5204 triggered on the HV signal). The highest probability of micro-discharge onset occurs for positive AC voltages between 6.6 and 7 kV, while for negative AC voltages, the best conditions occur between −8.5 and −9.5 kV.
Figure 3. Opto-electrical characteristics of filamentary CSDBD micro-discharges produced in humid air during positive and negative AC half-cycles, respectively. The discharge parameters were as follows: uAC = 22 kV peak-to-peak, fAC = 5 kHz, fM = 500 Hz, Q = 0.1 l min−1. Acquired as an average of 1024 samples triggering either on the HV signal (a), (b) or on the PMT signal (c), (d).
Download figure:
Standard image High-resolution imageFurther insight can be obtained by analysing the voltage, current and PMT waveforms for micro-discharges characterised by onset at the defined HV amplitude. Examples of such waveforms are shown in figure 3(c) and (d) (obtained by employing the A → B sequence trigger mode of the DPO5204). Figure 3(c) captures micro-discharges occurring at a HV level of 6.33 kV and causing sudden distortion of the regular AC waveform, characterised by a drop in the voltage of Δu+ ≅ 45 V. Similar behaviour can be observed during the negative half-cycle, as shown in (d) for an HV level of −9.18 kV and Δu- ≅ 15 V. In both cases, the duration of the current pulses is very short (≈5 ns) and the PMT signal is slightly delayed (a shift of ≈25 ns caused by the time response of R2949).
Since the CSDBD electrode with HV coaxial cable represents a ≈50 pF capacitor in the AC HV circuit, the voltage drop Δu+/ Δu− then reflects the variation in the energy stored in the capacitor. Assuming that the energy lost from the capacitor correlates with the energy dissipated in the discharge filament, we can roughly estimate the maximum mean energy released in a single micro-discharge filament. In the case of the events captured in figure 3(c) and (d), the maximum of estimated energy is about 15 ± 5 and 6.5 ± 2.2 μJ per filament for the events occurring during the positive and negative half-cycles, respectively.
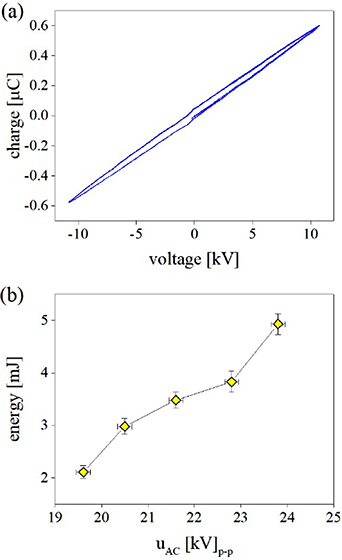
A precise determination of average energy per burst released by the discharge was made by inserting a non-inductive capacitor (C = 0.47 μF) into the ground lead to determine the transferred charge [45]. Figure 4(a) shows characteristic charge-voltage parallelograms (Lissajous figures) acquired under the discharge conditions used for the treatment. Figure 4(b) illustrates the dependence of the average energy per burst on the amplitude (peak-to-peak) of the applied HV waveforms. All treatment experiments were performed under discharge conditions characterised by an average energy of ∼3.5 mJ per burst, which corresponds to an average power of ∼1.7 W. Based on the CSDBD geometry and gas flow, the estimated average surface power density of ∼1.5 W cm−2 and energy density of ∼0.3 Wh l−1 fall into a range of conditions that are suitable for the efficient generation of ozone with minor concentrations of nitrogen oxides by surface streamers in air [45, 46].
Figure 4. Electrical characteristics of filamentary CSDBD micro-discharges produced in humid air. The transferred charge-voltage characteristics in (a) were obtained by inserting a non-inductive measuring capacitor (0.47 μF) into the grounding lead at uAC = 22 kV (peak-to-peak). The variation in the total energy (released during one HV burst composed of four AC cycles) with the amplitude of the applied HV AC waveform uAC is shown in (b). Results were obtained as an average over 1024 samples triggering on the HV signal. Other discharge parameters were as follows: fAC = 5 kHz, fM = 500 Hz, Q = 0.1 l min−1.
Download figure:
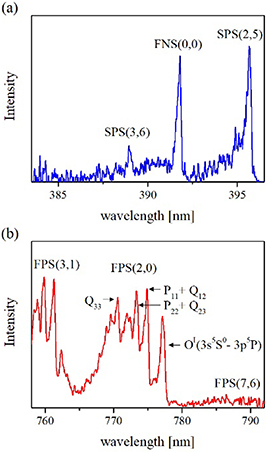
Standard image High-resolution imageThe time-averaged emission spectra produced by the CSDBD filaments and illustrated in figures 5 and 6 show all the major characteristics of streamer-based discharges [47, 48]. The spectra of filamentary CSDBD revealed strong bands of the second positive system (SPS) ( ) of N2 and weak first negative system (FNS) (
) of N2 and weak first negative system (FNS) ( ) of N2+ in the UV spectral range, while in the vis-NIR range, we observed characteristic sequences of bands of the first positive system (FPS) (
) of N2+ in the UV spectral range, while in the vis-NIR range, we observed characteristic sequences of bands of the first positive system (FPS) ( ) of N2 and atomic lines of neutral oxygen OI.
) of N2 and atomic lines of neutral oxygen OI.
Figure 5. Characteristic emission spectra of axially symmetric CSDBD produced in humid air. Data were acquired from the whole active CSDBD surface as an average over 104 AC HV cycles, using an input slit width of 100 μm and a diffraction grating of 300 G mm−1 (intensities of the UV and vis-NIR parts not to scale). Discharge parameters were as follows: uAC = 22 kV peak-to-peak, fAC = 5 kHz, fM = 500 Hz, Q = 0.1 l min−1. Emission spectra were not corrected for the wavelength-dependent detector sensitivity.
Download figure:
Standard image High-resolution imageFigure 6. Characteristic emission spectra of axially symmetric CSDBD produced in humid air. Data were acquired from the whole active CSDBD surface as an average over 5 × 105 AC HV cycles, using (a) an input slit of 50 μm and a diffraction grating of 3600 G mm−1 and (b) an input slit of 100 μm and a diffraction grating of 1200 G mm−1 (other conditions as in figure 5). Emission spectra were not corrected for the wavelength-dependent detector sensitivity.
Download figure:
Standard image High-resolution imageThe partially-resolved rotational structures of the SPS, FNS and FPS bands displayed in figure 5 were analysed by means of the synthetic models detailed in [47, 49, 50]. The FNS(0,0) band can be fitted using a given instrumental function by fixing the rotational temperature at 315 ± 20 K. The rotational temperature of the N2(B3Πg, v = 2) vibronic state was determined using a peak method described in [47, 50]. This method utilises the temperature-sensitive ratio of three selected sub-band heads; in the case of the FPS(2,0) band, these are formed from the P11 + Q12, P22 + Q23 and Q33 branches (indicated in figure 6(b)), and the Trot determined from the time-averaged spectra reaches 325 ± 15 K.
3.2. Effect of air CSDBD plasma on B. subtilis spores
Membranes inoculated with the B. subtilis spores were exposed to reactive species, and the dependence of the number of surviving bacteria on the treatment time was studied under fixed treatment conditions (discharge power, CSDBD plasma-to-sample distance, fixed airflow through the discharge chamber, sample and air humidity). In this work, we tailored the discharge duty cycle and maintained constant airflow through the discharge in order to keep the gas temperature low enough to exclude partial thermal inactivation. The rotational temperature obtained from two different excited states of molecular nitrogen indicated a low gas temperature in the plasma filaments (about 325 K), and hence also a low temperature at the position of the sample with the spores. The distribution of spores on the filter was homogeneous, as verified by the SEM images [51] (a heterogeneous distribution of the spores on the sample holder can cause the formation of cells refuges, and can shield a certain fraction of spores from the reactive species).
We verified that the airflow through the discharge led to progressive drying (thereby changing the treatment conditions) of the bacterial samples. In order to maintain the humidity of exposed samples during the whole exposure, it was necessary to add 10 μl of sterile water to the sample holder prior to switching on the discharge. Losses due to the evaporation of water from filter samples under the given exposure geometry were verified gravimetrically. The rate of evaporation losses in the case of dry airflow through the chamber with the discharge switched on was about 3.9 μl min−1 (compared with 2.9 μl min−1 with the discharge off). In the case of humid airflow with the discharge switched on, the evaporation rate was about 2.1 μl min−1 (compared with 0.22 μl min−1 with the discharge off). This means that in the case of the discharge with humid airflow and 10 μl of sterile water, the filter with bacterial samples becomes completely dry within few minutes, completely changing the initial treatment conditions (wet → dry).
Counting bacterial populations reveals a roughly biphasic shape for the inactivation kinetics [52], before a plateau is reached (after approximately 2 min exposure) with as much as 97% (1.5 log) of spores inactivated during plasma-assisted treatment of humidified samples (figure 7). The D-value (inactivation 1.0 log10) determined in this work was 1.17 min, which is very similar to the D-value of 1.21 min reported by Hertwig et al [14], or the value found by Wang et al [15], which was even below 1 min. It is clear that the rate of inactivation slows down significantly with progressive drying of the samples. This is indirect evidence for the important role of the dissolved reactive species. It is also important to emphasise here that due to the technique of the amplitude modulation technique used for the driving AC HV (duty cycle of 0.4), a two-minute exposure means 48 s of plasma ON and 72 s of plasma OFF. Furthermore, assuming monolayer exposure of the bacteria and considering the mean discharge power, we can estimate an upper limit of 2.5 × 10−6 mJ CFU−1 (or 1.5 × 1010 eV CFU−1) for the mean energy necessary to inactivate one bacterium.
Figure 7. Resistance of spores of B. subtilis to exposure by reactive species produced by the multiple CSDBD. B. subtilis was filtered through a polycarbonate filter, and the filter containing bacteria was then moistened with 10 μl of sterile water just before exposure. CSDBD was driven in humid synthetic air, with a fixed distance between the electrode and sample of 3 mm. The discharge parameters were as follows: uAC = 22 kV peak-to-peak, fAC = 5 kHz, fM = 500 Hz, Q = 0.1 l min−1.
Download figure:
Standard image High-resolution imageHertwig et al [14] treated inoculated glass beads by diffuse coplanar surface barrier discharge, which was continuously excited (20 kV peak-to-peak at 15 kHz). The discharge chamber was continuously shaken to obtain homogeneous treatment. Consequently, at any instant, part of the inoculated surface of each bead was in direct contact with the discharge filaments, while the other part of the surface was treated indirectly (as in the case of this work). The authors claim that the air-plasma temperature did not exceed 63 °C, even though the treatment was performed in the static gas at a power input of 350 W (compared with the average power of ∼1.7 W used in this work). However, they measured only the average gas temperature with a fiber-optic temperature sensor (based on a GaAs crystal with a response time of 2 s), which cannot reflect fast temperature variations inside plasma filaments on a μs-ms timescale. It is therefore very likely that the inoculated beads were locally in contact with plasma filaments characterised by significantly higher temperatures, and partial thermal inactivation cannot be excluded, especially at longer exposures (3.5 log10 inactivation for 7 min exposures).
Wang et al [15] used volume DBD with air gap of 1.5 mm (continuous AC discharge, 14 kV peak-to-peak at 60 Hz) to treat directly inoculated microscope glass cover slips. These authors used an infrared thermometer to measure the temperature of inoculated cover slips after the DBD treatment, which, again, cannot be used as proof of the gas temperature of DBD plasma filaments during the treatment. Due to the much longer treatment times (up to 30 min) under static gas conditions, partial thermal inactivation cannot be excluded, as in [14]. In fact, Wang et al [15] report 5.5 log10 inactivation for 3 min exposures, which clearly indicates that the temperature in a volume DBD configuration was even higher than that reported by Hertwig et al [14].
When treating homogeneously distributed spores, one might expect linear kinetics of inactivation; however, multiphasic kinetics [52] is observed instead (see figure 7). Bayliss et al suggested that a biphasic kinetics is caused by the response of the bacterial population, which tend to resist to the plasma treatment. This response includes rearranging cells into aggregates, preventing plasma-generated species from reaching the individual cells [32]. On the other hand, Moisan et al proposed that the plasma inactivation of B. subtilis spores displayed two or three different phases in the survival curve, mainly caused by a shift in the plasma-generated processes predominating at each given moment. According to the author, the complex shape of inactivation curve was probably caused by the shift from the UV caused to reactive species killing [53]. The change in the dominant plasma-assisted processes (wet treatment → dry treatment) seems to be the cause of the multiphasic kinetics observed in this work. The effects of plasma-generated UV and reactive species are discussed later in the text.
3.3. The effect of reactive species to spore inactivation
Plasma-induced inactivation may be caused mainly by UV light, heat, chemically reactive species and energetic charged particles [54]. Concerning the possible effects of UV light on spore inactivation (via DNA damage enabled by UV light), CSDBD driven in wet air strongly emits UV-A photons (315–380 nm), less intense UV-B photons (280–315 nm) and negligible UV-C photons (bellow 280 nm), as evidenced in figure 5. Nevertheless, both UV-A and UV-B photons are able to induce various forms of damage in bacterial DNA, such as spore photoproducts or cyclobutane pyrimidine dimers [55, 56]. However, we do not expect any significant contribution from UV irradiation of the spores, since a strong source of UV-C is missing under humid air discharge conditions; in addition, a significant part of the UV-B radiation (produced by the N2-SPS emission) is absorbed by O2, O3 and H2O species between the discharge filaments and the remote (3 mm) spores. In a prior study, we demonstrated that the contribution of UV light generated by the air CSDBD has a negligible antibacterial effect [36]. In addition, the observation of a significant slow-down in the inactivation kinetics after 2 min provides independent evidence of the negligible influence of UV photons in the present work. An earlier study by Deng et al [57] tested the influence of UV emission produced by an atmospheric-helium plasma plume. These authors performed experiments with the spore samples that were protected from all plasma products except for UV photons (given in this case by the NO-γ bands in UV-C, N2-SPS and OH emission at 310 nm in UV-B, and N2-SPS and N2+-FNS in UV-A [47]), and they concluded that the ROS generation in a comparable atmospheric helium–oxygen plasma plume was the primary process of killing bacteria, whereas the contributions from UV photons (inspected without an oxygen admixture), charged particles and heat were of minor importance. Since the emission in a helium plume is a significantly stronger source of UV-C and UV-B compared with the SPS produced by the CSDBD in humid air, we must expect negligible UV-assisted inactivation under the present conditions.
It has been proposed that the contribution of reactive species to the plasma-caused bacterial inactivation is significant [58, 59]. We tried to identify species that might participate in microbial inactivation under the present conditions. Previous studies have concluded that the effects of post-discharge chemistry, including ozone, nitrates, nitrites and hydrogen peroxides, might significantly contribute to plasma-assisted inactivation [36, 44, 60]. The main focus was on the detection of the chemically reactive products generated by the discharge and transferred to the liquid.
As a result of the experimental procedure used here, some residual liquid water remains on the filter, and we must expect the formation of H2O2 and NO2− in the wet environment surrounding the cells. The residual water was introduced by moistening the filter with 10 μl of sterile water in addition to the humidity present in the air flux. Unfortunately, such a small volume was insufficient for the H2O2 and NO2− analysis, and we therefore performed a complementary measurement using a larger H2O volume. We determined the formation of H2O2 and NO2− in 2.5 ml of deionised water, placed under the discharge at the same distance as the filter with the spores. The concentration of hydrogen peroxide and nitrite ions formed in exposed water increased with the duration of treatment (figure 8). Moreover, the pH of the exposed water decreased from 6 to 3.7 after 6 min of treatment. However, it was found that the concentration of RONS dissolved in water depends on the volume and the area of the water surface in contact with the plasma [61, 62]. Hence, we must expect much higher concentrations of RONS dissolved in 10 μl of water during the treatment of bacterial samples. This also means that dissolved RONS are expected to contribute significantly to the inactivation of humid samples.
Figure 8. Concentrations of hydrogen peroxide and nitrites measured in the deionised water due to reactive species produced by the CSDBD. The discharge parameters were as follows: uAC = 22 kV peak-to-peak, fAC = 5 kHz, fM = 500 Hz, Q = 0.1 l min−1. Determination of the hydrogen peroxide and nitrite ions was carried out by the tytanil sulphate and Griess reagent, respectively.
Download figure:
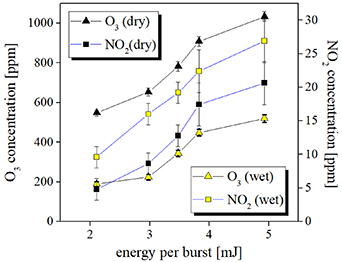
Standard image High-resolution imageGaseous ozone and NxOy species were monitored at the CSDBD reactor output port in both dry and humid air without bacterial samples. The results corresponding to an airflow rate of 0.5 l min−1 and at various energies are shown in figure 9. At the discharge conditions used for the treatment, the concentration of produced NO was always below the detection threshold, for the mass flow Q in the range 0.5–1 l min−1, while some PPM of NO2 were always detected in both dry and humid air. Concentrations of ozone of several hundreds of PPM were obtained, with notably higher densities obtained in dry air. Taking into consideration the very high ratio of O3/NOx ≫ 1, we can expect that the CSBDB produces mainly O3 with relatively high energy efficiency for a flow rate of Q = 0.1 l min−1 in humid air. The findings suggest that the CSDBD reactor works in ozone 'mode' which should favour bactericidal effects [63]. The observation of NO2– formation confirms the reaction via which nitrogen oxides generated in the gas phase dissolve in the aqueous phase [38]. Nitrites can also be formed by chain reactions, directly in the humid-air plasma, and subsequently transformed into liquid [64].
Figure 9. Concentrations of ozone and nitrogen dioxide produced by the CSDBD in dry/wet synthetic air and measured at the exit of the reactor. Experimental conditions are as in figure 4(b). Determination of the ozone and nitrogen dioxide was carried out without biological samples inserted in the gap at an airflow rate of 0.5 l min−1.
Download figure:
Standard image High-resolution imageAlthough spores are extremely resistant to various chemicals, they are susceptible to nitrous acid, ozone and hydrogen peroxide, which we detected in the plasma-treated water. The plasma-assisted generation of hydrogen peroxide may make a contribution to the killing of spores; however, the mechanism via which hydrogen peroxide may affect the spore cell remains uncertain. It has been shown that small acid-soluble proteins (SASPs) play a significant role in the resistance of spores to hydrogen peroxide, and it is therefore likely that hydrogen peroxide kills the spore likely at other sites than DNA and that the mechanism is different from the killing of vegetative cells [17, 21, 24]. Melly et al [65] proposed that spores killed by hydrogen peroxide maintain their membrane permeability, blocking the core contents from leaking out. This persistent membrane stability may be caused by the fact that hydrogen peroxide attacks polyunsaturated fatty acids as the usual target in the membrane, while the spore has an extremely low level of polyunsaturated fatty acids. Additionally, it assumes that the proteineous coat acts as a barrier or target to lower H2O2 concentration which can react with the core structure [23, 66]. The effect of hydrogen peroxide is likely to be caused by defects in a healthy metabolism [65]. Based on the investigations mentioned above, we are of the opinion that plasma-generated hydrogen peroxide contributes to spore killing, although the specific mechanism is unknown.
Furthermore, nitrous acid, which is generated in liquid treated by plasma above the surface, is a well-known mutagen. The study of Tennen et al [67] evidenced high levels of DNA damage after the exposure of spores to nitrous acid. We therefore expect that nitrous acid formed by plasma may cause the same defects in spores, although further specific tests of the extent and type of this DNA damage need to be carried out. Moreover, a systematic study by Hertwig et al [14] investigated the effects of plasma on isogenic mutants of the B. subtilis strain without (i) genes encoding SASPs, (ii) DPA-deficient spores, and (iii) spore outer protein. It appeared that Bacilllus spores without SASPs were inactivated more effectively.
Spore decontamination was reported after treatment by ozone and peroxynitrous acid, which are both commonly generated by discharge reactions [68, 69]. The formation of peroxynitrous acid in the post-discharge chemistry involves hydrogen peroxide and nitrites in the plasma treated water [38]:
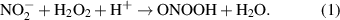
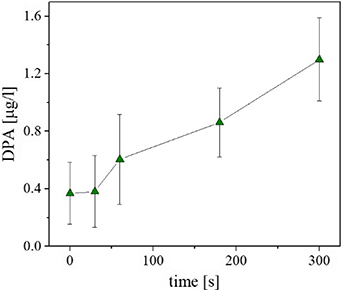
The formation of high concentrations of ozone was detected in the output from the reactor. Ozone and peroxynitrous acid affect spores by damaging inner membrane of the cell, and this can be supported by observing DPA release. Although the treatment of spores with peroxynitrous acid and ozone does not cause straightforward leaking out of DPA [68, 69], the subsequent heat treatment initiates the loss of DPA. We observed an increasing concentration of free DPA with an increase in the time of treatment, as illustrated in figure 10. We therefore suggest that DPA release can contribute significantly to plasma-assisted inactivation, as also observed in other studies [15, 16, 70].
Figure 10. Release of DPA after plasma treatment. The experimental conditions were as follows: multiple micro-discharge in synthetic humid air, other conditions as in figure 7. The determination of DPA was carried out according to Warth [41].
Download figure:
Standard image High-resolution imageFinally, we detected DNA leakage into the surrounding suspension (an increase of 1.96 μg ml−1 after 5 min), although the detected concentration and increase was very small, probably due to the extremely good protection of the spores in the area around the DNA. Nevertheless, an increase in the free intracellular components, including the DNA, can be considered as an important sign of a leaking membrane, cortex, and/or coat structure. Thus, free DNA occurring in the spore suspension after plasma treatment may be caused by the destabilisation of the crucial biochemical structure of the spore.
4. Conclusions
The aim of the present study was to investigate the effects of multiple surface streamer micro-discharge on spores of B. subtilis. The results were obtained using an AC amplitude-modulated CSDBD driven in atmospheric-pressure humid air, characterised by low-temperature plasma filaments produced at a fixed distance of 3 mm from bacterial samples, which inhibited both thermal and UV inactivation of spores.
Before the treatment, the spores were prepared via a universal experimental protocol [26, 27]. Bacterial monolayers were deposited on a filter membrane to prevent the formation of clumps or cellular shelters (inhibiting the effects of the plasma) and then exposed to reactive species produced by the CSDBD working in the 'ozone' mode (O3/NOx ≫ 1) at an average power of 1.7 W (surface power density of ∼1.5 W cm−2 and energy density of ∼0.3 Wh l−1). The DBD reactor was operated at a duty cycle of 0.4, which appears to be sufficient to maintain the gas temperature at a low enough level at the given gas flow. We used emission spectra characteristics to determine the gas temperature in the gap, which was no higher than 325 ± 15 K.
After exposure, spore samples exhibited multiphasic kinetics of inactivation for up to 300 s of plasma treatment, with an estimated energy cost of 2.5 × 10−6 mJ CFU−1 (or 1.5 × 1010 eV CFU−1). Initial fast biphasic decrease (up to 2 min) of surviving spores was followed by a slowly decreasing plateau (up to 5 min). This behaviour was very likely to have been caused by the progressive evaporation of water from the filter containing spores. The initial efficient wet treatment of spores with the participation of various RONS dissolved in water was replaced by a less efficient dry treatment by species occurring in the gas phase. The measurements of the reactive species indicate that the most important processes involved in the plasma-assisted inactivation of spores are driven by reactive species (O3, H2O2, NO2–) and the effect of UV light is very likely to be negligible.
We assume that one of the most significant pathways contributing to the spore inactivation involves multiple forms of damage affecting the spore integrity, leading to changes in the permeability of the inner spore membrane with a subsequent release of the DPA and other intracellular components (such as DNA). This conclusion is in agreement with the results of other studies [14–16]; however, these studies cannot exclude the effects of temperature enhancement caused by employing a DBD discharge in continuous mode and under static gas conditions, which necessarily lead to higher surface and bulk gas temperatures compared with the present work.
Acknowledgments
This work was supported by the TACR (project No. TA03010098) and Czech Science Foundation (project No. 15-04023S). E D and A K acknowledge support from MEYS (project LD13010, VES13 COST CZ, COST Action MP 1101).