Abstract
Purpose
The incidence of acute myocardial infarctions (AMI) shows circadian variation typically peaking during morning hours with a decline at night. However, this variation does not occur in patients with diabetes mellitus (DM). The night’s decline of AMI may be partially explained by melatonin-related platelet inhibition. Whether this effect is absent in diabetic patients is unknown. The aim was to study the effect of melatonin on in-vitro platelet aggregation in healthy individuals and patients with type 2 DM.
Methods
Platelet aggregation was measured in blood samples from healthy individuals (n = 15) and type 2 DM patients (n = 15) using multiple electrode aggregometry. Adenosine diphosphate (ADP), arachidonic acid (ASPI) and thrombin (TRAP) were used as agonists. Aggregability for each subject was tested after adding melatonin in two concentrations.
Results
In healthy individuals, melatonin inhibited platelet aggregation in both higher (10–5 M) and lower concentrations (10–9 M) induced by ADP, ASPI, and TRAP (p < 0.001, p = 0.002, p = 0.029, respectively). In DM patients, melatonin did not affect platelet aggregation in both concentrations induced by ADP, ASPI, and TRAP. Melatonin decreased platelet aggregation induced by ADP, ASPI, and TRAP significantly more in healthy individuals compared to patients with DM. (p = 0.005, p = 0.045 and p = 0.048, respectively).
Conclusion
Platelet aggregation was inhibited by melatonin in healthy individuals. In-vitro antiplatelet effect of melatonin in type 2 DM patients is significantly attenuated.
Similar content being viewed by others
Introduction
Acute myocardial infarction (AMI) is a leading cause of mortality and morbidity in the developed world. Depending upon the infarction size, 30-day mortality is up to 6.5% [1,2,3]. From the surviving patients, 10% will die within 12 months and almost half of the patients will require rehospitalization within one year [4, 5].
The incidence of AMI shows a circadian variation that peaks during morning hours continuously declines in the afternoon, and reaches a trough during the evening and night-time. The increased morning incidence of AMI is most likely caused by a rise in blood pressure, heart rate, vascular tone and prothrombotic activity [6,7,8,9]. Interestingly, in the population of patients with diabetes mellitus (DM), circadian variation of AMI is absent [10]. We hypothesized this could be caused by the inability of melatonin to inhibit platelets aggregation in DM.
Melatonin is an endogenous hormone released primarily by the pineal gland and is one of the key components of the human circadian system [11, 12]. Melatonin directly or indirectly affects many physiological functions including the immune system, body temperature, foetal development, metabolism, coagulation [13,14,15,16,17] and platelet aggregation [18,19,20]. Evidence shows that genetic variants in the melatonin receptor as a result of single nucleotide polymorphisms are associated with atherosclerosis and the risk of myocardial infarction (MI) [21,22,23]. The relationship between melatonin and DM is also the subject of extensive research. Melatonin supplementation has been shown to improve insulin resistance, leptin resistance, hyperinsulinaemia, hyperglycaemia and reduce HbA1c levels. Low levels of melatonin secretion were able to predict the onset of Type 2 DM in women [24] and melatonin has been also studied as a potential drug in the therapeutic management of diabetic patients [25].
It is not known which platelet aggregation pathways are impaired by melatonin and if this effect is attenuated in DM. We designed a study to evaluate the effect of melatonin on platelet aggregation activated by arachidonic acid, adenosine diphosphate and thrombin in the blood of healthy individuals and in patients with type 2 DM.
Methods
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the National Cardiovascular Institute, Bratislava, Slovakia. Written informed consent was obtained from all participants.
Study population
Fifteen consecutive healthy adult individuals scheduled for blood donation were enrolled in the control normoglycemic group and 15 consecutive adult outpatients with type 2 DM on insulin were enrolled in the DM group. Exclusion criteria for both groups were treated with any antiplatelet, anticoagulant, or anti-inflammatory drug, smoking, present cancer, acute or chronic infectious disease, renal disease, pregnancy, history of any thrombotic cardiovascular disease, history of any platelet disorder or bleeding disorder and platelet count < 120 × 109/L.
Laboratory methods
In all participants, peripheral, fasting blood was taken from an antecubital vein in the morning after 30 min rest in a seating position. Blood was collected during the daytime when melatonin concentrations are very low (below 1 pg mL−1) [26]. These levels are negligible compared to night-time levels, which we mimicked in our study.
Blood for platelet counts was collected in 3.0 mL tubes containing K2EDTA and assessed by an automated haematology analyser SYSMEX XT 4000i.
Blood for platelet aggregation analysis was collected in 3.0 mL tubes containing hirudin and stored at room temperature for a minimum of 30 min and a maximum of two hours before analysis. Subsequently, blood samples were aliquoted and incubated with saline or melatonin in two different concentrations (10–5 and 10–9 M, respectively) for 10 min.
Platelet aggregation analysis was performed by multiple electrode aggregometry using the impedance-based Multiplate® Analyzer (Roche, Mannheim, Germany). Arachidonic acid 15 mmol/L (ASPI), Adenosine diphosphate 0.2 mmol/L (ADP) and thrombin-receptor-activating-peptide (TRAP-6) 1 mmol/L (TRAP) were used as agonists (ASPItest, ADPtest and TRAPtest, Roche, Mannheim, Germany). Platelet aggregation levels are expressed as area under the curve (AUC) in Units (U) derived from the older AU * min (1U = 10 AU * min). Sample preparation and pipetting were done under standard laboratory conditions in laboratories in the Faculty of Natural Sciences, Comenius University, Bratislava. Measurement analysed by Multiplate® Analyzer is dependent on the hematocrit level and platelet count such that extreme values of these parameters may result in an imprecise assessment of platelet function. However, no extreme values in both hematocrit levels and platelet count were detected in any sample.
Statistical methods
Continuous variables are presented as sample means and standard deviations. The normality of data was assessed using a Shapiro–Wilk test and visually inspected on Q–Q plots. Repeated measures ANOVA was used to analyse concentration differences for each group and agonists separately. Student’s t test was used to compare differences in platelet aggregation response induced by ADP, ASPI, and TRAP with saline vs melatonin (10–5 M). Mixed linear model regression was used to analyse the effect of covariates (study group, age, sex and melatonin concentration) as well as the interaction of group (diabetic and control) and melatonin concentration on the platelet aggregation levels for each agonist separately.
Data were analysed using Python version 3.7.12 (https://www.python.org/) with appropriate libraries (for statistical analyses pingouin package version 0.5.0: https://pingouin-stats.org/).
Sample size calculation
Platelet aggregation in the healthy population measured by Multiplate analyser was 68.6 ± 20.12 for ADP, 72.3 ± 18.08 for ASPI and 104.6 ± 19.60 for TRAP, respectively [27]. Expected reduction in platelet aggregation in response to melatonin is 30% in healthy individuals and 0% in diabetic patients [18]. With a minimal relevant difference of 20 U, a level of significance of 5% (alpha) and a power of 90%, (1-beta) we needed 13 sample pairs for ADP. With a minimal relevant difference of 22 U, a level of significance of 5% (alpha) and a power of 90%, (1-beta) we needed 10 sample pairs for ASPI. And with a minimal relevant difference of 31 U, a level of significance of 5% (alpha) and a power of 90%, (1-beta) we needed seven sample pairs for TRAP.
Results
Our study was composed of two groups. The healthy control group (n = 15) included 11 males and 4 females with a mean age of 31.67 (ranging from 19 to 44, SD ± 7.28). Patients in this group had no relevant medical history.
Diabetic group (n = 15) included 4 males and 11 females with a mean age of 72.47 (ranging from 59 to 89, SD ± 10.12). Patients in this group had no relevant medical history. There was a statistically significant difference in age (p < 0.001) and sex (p < 0.001) between the groups (Table 1).
Since the data for all markers were normally distributed (ADP: W = 0.98, p = 0.25; ASPI: W = 0.99, p = 0.88; TRAP: W = 0.99, p = 0.67), we decided to use parametric statistical tests for subsequent analyses.
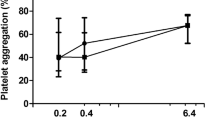
In healthy individuals, melatonin significantly inhibited platelet aggregation both in higher (10–5 M) and lower concentrations (10–9 M) induced by ADP (Fig. 1), ASPI (Fig. 2), and TRAP (Fig. 3). Repeated measures ANOVA demonstrated statistically significant reductions (ADP: p < 0.001, ASPI: p = 0.002, TRAP: 0.029).
The difference in platelet aggregation response between healthy individuals (control) and diabetic patients induced by ADP: saline, melatonin 10–5 and melatonin 10–9 concentrations. Data are presented as mean with 95% confidence interval. Control group—n = 15, diabetic group—n = 15. ADP adenosine diphosphate, AUC area under the curve
The difference in platelet aggregation response between healthy individuals (control) and diabetic patients induced by ASPI: saline, melatonin 10–5 and melatonin 10–9 concentrations. Data are presented as mean with a 95% confidence interval. Control group—n = 15, diabetic group—n = 15. ASPI arachidonic acid, AUC area under the curve
The difference in platelet aggregation response between healthy individuals (control) and diabetic patients induced by TRAP: saline, melatonin 10–5 and melatonin 10–9 concentrations. Data are presented as mean with a 95% confidence interval. Control group—n = 15, diabetic group—n = 15. TRAP thrombin, AUC area under the curve
In samples from patients with DM melatonin did not affect platelet aggregation both in higher (10–5 M) and lower concentrations (10–9 M) induced by ADP (Fig. 1), ASPI (Fig. 2), and TRAP (Fig. 3). Repeated measures ANOVA demonstrated no statistically significant reduction (ADP: p = 0.579, ASPI: p = 0.871, TRAP: p = 0.757).
The difference in platelet aggregation response induced by ADP, ASPI, and TRAP with saline vs melatonin (10–5 M) was significantly higher in healthy individuals compared to patients with DM (p = 0.005, p = 0.045 and p = 0.048, respectively) (Fig. 4).
Mixed linear model regression models for TRAP (Table 2), ASPI (Table 3) and ADP (Table 4) showed a statistically significant effect of the study group (control vs. diabetic, p < 0.001, p = 0.014 and 0.006, respectively), 10–5 melatonin concentration (p = 0.01, p = 0.001 and p < 0.001, respectively), 10–9 melatonin concentration (p = 0.002, p = 0.001 and p = 0.007, respectively) and age (p < 0.001, p = 0.016 and p = 0.009, respectively) on platelet aggregation response. There was a statistically significant interaction between study group and 10–5 melatonin concentration (p = 0.035, p = 0.037 and p = 0.02) on platelet aggregation response. There was a statistically significant interaction between the study group and 10–9 melatonin concentration on platelet aggregation response for TRAP and ASPI (p = 0.041 and p = 0.037, respectively) but not for ADP (p = 0.098). There was no statistically significant effect of sex on platelet aggregation response induced by TRAP, ADP and ASPI (p = 0.618, p = 0.857 and 0.491, respectively).
Discussion
Our in vitro study demonstrated that melatonin significantly attenuates platelet aggregation induced by arachidonic acid, adenosine diphosphate and thrombin-receptor-activating-peptide in healthy patients’ whole blood. Blood was collected during the daytime when melatonin concentrations are very low, (below 1 pg mL−1). These concentrations are negligible compared to night-time levels, which we mimicked in our study. Moreover, published studies show that daytime melatonin levels do not significantly differ between nondiabetic individuals and diabetic patients [26]. This was true for higher (10–5 M) and lower (10–9 M) concentrations which are similar to the physiological concentrations in human blood [28]. In the whole blood of diabetic patients, melatonin was not associated with statistically significant differences in platelet aggregation. Finally, the attenuation of whole blood platelet aggregation induced by melatonin was significantly higher in healthy people compared to diabetic patients.
In the bivariate analysis, there was a statistically significant difference in both age and sex between the two study groups. Mixed linear model regression was used to analyse the effect of these covariates on platelet aggregation induced by ADP, ASPI and TRAP. This analysis confirmed attenuated response to melatonin in diabetic patients in all experiments except for lower (10–9 M) concentration for ADP. Sex was not a significant contributor contrary to previously reported data. A review study by Carazo et al. shows that out of 78 reviewed papers 68 reports a sex-related difference in platelet aggregation [29]. Higher platelet reactivity in women is probably affected via multiple COX‐1–dependent and COX‐1–independent pathways [30]. On the other hand, age was a significant contributor to platelet aggregation also in this multivariate analysis. The effect of aging on platelet aggregation is complex and probably influenced by many factors including oxidative stress, age-related plasma membrane modifications, alterations in platelet-serotonin system, vascular prostaglandin secretion, transcriptome, hormonal changes and the effect of coexisting diseases [31].
Previous studies reported that melatonin inhibits platelet aggregation that is induced by ADP or ASPI [19, 20], and we demonstrated that melatonin also inhibits aggregation induced by thrombin. However, there are no melatonin receptors in thrombocytes [32], and the antiplatelet mechanism of melatonin is unknown. The three most important activators of platelet aggregation are ASPI, ADP and thrombin which were used in our study as prothrombotic inductors. Because melatonin in healthy people attenuates aggregation in all three of these activators, there is little likelihood that melatonin exerts its antiplatelet effects via one of these pathways. Besides known effects of melatonin on platelet aggregation a study on animal models by Hajam et al. demonstrated also that melatonin treatment restores impairments in the antioxidative system, serum electrolytes, cellular total protein, glycogen content and histoarchitecture of liver and kidney cortex caused by diabetes. The novelty of our study lies in the previously undescribed altered effect of melatonin in diabetic patients [33].
Except for triggering melatonin receptors, melatonin also activates proliferator-activated receptor (PPAR) α and γ [34,35,36,37]. It is known that PPAR stimulation causes increased intraplatelet cAMP, negative regulation of αIIbβ3 integrinand subsequent inhibition of platelet aggregation [38, 39].
One study reports that melatonin suppresses platelet aggregation via activation and restoration of PPARγ in platelets, which play an important role in FUNDC1‐required mitophagy, mitochondrial energy production, platelet hyperactivity, and cardiac I/R injury [40]. This might explain the antithrombotic effects of melatonin in healthy participants.
In our in vitro study melatonin did not affect platelet aggregation in patients with DM. The night-time antiplatelet mechanism of melatonin is missing in DM patients and might be responsible for their absence of AMI circadian variation.
One explanation for this phenomenon is the alteration of the PPAR signalling pathway seen in diabetes and hyperglycaemia. The transcriptional network mediated by FoxO1/PPARγ functions as a key element in pancreatic β-cell adaptation to metabolic stress with important regulatory control over glucose and mitochondrial metabolism, prodifferentiation, incretin effects, and β-cell compensation to obesity and insulin resistance. Failure of this response is responsible for the onset or exacerbation of diabetes. Furthermore, excessive expression of pro-inflammatory cytokines suppresses PPARγ activity causing abnormalities of the wnt/β-catenin, lysosomal acid lipase, plasminogen activator system, inflammatory and cell cycle pathways [41, 42].
This hypothesised relationship is further supported by the results from clinical trials of PPAR agonists. For example, PPARγ activation by pioglitazone reduced the incidence of AMI or stroke in patients with insulin resistance however, according to the prespecified sub-analysis the beneficial effect was present especially in patients with lesser grade insulin resistance (HOMA-IR < 4.6) and lower glycated haemoglobin concentrations (HBA1C < 5.7%) [43]. On the other hand, when rosiglitazone was given to patients with DM, a significant increase in AMI risk observed [44]. A similar situation was observed with fibrates which are PPARα agonists. In patients without DM, gemfibrozil showed significant reductions in MI and stroke [45, 46]. However, in patients with DM fenofibrate had no effect on these thrombotic events[47, 48].
This is the first study to demonstrate in vitro that the antiplatelet effect of melatonin in patients with type 2 DM is significantly attenuated, possibly explaining their absence of circadian variation in AMI incidence. Whether this finding contributes to the etiopathogenesis of the prothrombotic state in patients with DM merits further research. Understanding the exact mechanism of platelet resistance to melatonin in diabetic patients would permit a better understanding of the disease pathophysiology and also the consideration of new therapeutic and diagnostic options such as therapeutically targeting the dysfunctional signalling pathways in diabetic patients or testing the degree of resistance to melatonin to stratify patient risk. A precise understanding of PPAR pathway and the influence of individual signalling molecules would allow using PPAR agonists to reduce the risk of MI and stroke in precisely defined patient groups.
Study limitations
This study was performed as an in vitro experiment and although physiological concentration (M-9) of melatonin was used the results cannot be directly extrapolated to in vivo pathophysiology. Patients were not matched in the study groups therefore, other factors besides the presence of type 2 diabetes mellitus and age such as menstrual cycle, hormonal contraception, or differences in body mass index might have affected the platelet aggregability.
Data availability
Raw data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
References
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA et al (2018) Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72(18):2231–2264. https://doi.org/10.1016/j.jacc.2018.08.1038
Asano T, Mitsuhashi Y, Sachi M, Wakabayashi K, Yahagi K, Shinke T et al (2020) The impact of low diastolic blood pressure on 30-day mortality of patients with acute myocardial infarction. Eur Heart J. https://doi.org/10.1093/ehjci/ehaa946.1652
Oecd (2017) Health at a Glance 2017: OECD indicators. Mortality following acute myocardial infarction (AMI). OECD Publishing, Paris
Mechanic OJ, Gavin M, Grossman SA (2022) Acute Myocardial Infarction. In: StatPearls. StatPearls Publishing, Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL)
Jelic D, Mehmedbegovic Z, Milasinovic D, Dedovic V, Zobenica V, Zaharijev S et al (2019) P953Comparison of the original and updated ACTION risk scores for predicting in-hospital and one-year mortality in patients with acute myocardial infarction undergoing primary PCI. Eur Heart J. https://doi.org/10.1093/eurheartj/ehz747.0547
Takeda N, Maemura K (2016) Circadian clock and the onset of cardiovascular events. Hypertens Res 39(6):383–390. https://doi.org/10.1038/hr.2016.9
Van Laake LW, Lüscher TF, Young ME (2018) The circadian clock in cardiovascular regulation and disease: lessons from the Nobel Prize in Physiology or Medicine 2017. Eur Heart J 39(24):2326–2329. https://doi.org/10.1093/eurheartj/ehx775
Giles TD (2006) Circadian rhythm of blood pressure and the relation to cardiovascular events. J Hypertens Suppl 24(2):S11–S16. https://doi.org/10.1097/01.hjh.0000220098.12154.88
Scheer FA, Michelson AD, Frelinger AL 3rd, Evoniuk H, Kelly EE, Mccarthy M et al (2011) The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 6(9):e24549. https://doi.org/10.1371/journal.pone.0024549
Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA (2003) Circadian variation in the onset of myocardial infarction: effect of duration of diabetes. Diabetes 52(6):1464–1468. https://doi.org/10.2337/diabetes.52.6.1464
Stehle JH, Von Gall C, Korf HW (2003) Melatonin: a clock-output, a clock-input. J Neuroendocrinol 15(4):383–389. https://doi.org/10.1046/j.1365-2826.2003.01001.x
Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK et al (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19(1):91–102. https://doi.org/10.1016/s0896-6273(00)80350-5
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N et al (2017) Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol 15(3):434–443. https://doi.org/10.2174/1570159x14666161228122115
Feelisch M, Kolb-Bachofen V, Liu D, Lundberg JO, Revelo LP, Suschek CV et al (2010) Is sunlight good for our heart? Eur Heart J 31(9):1041–1045. https://doi.org/10.1093/eurheartj/ehq069
Kostovski E, Dahm AE, Iversen N, Hjeltnes N, Østerud B, Sandset PM et al (2011) Melatonin stimulates release of tissue factor pathway inhibitor from the vascular endothelium. Blood Coagul Fibrinolysis 22(4):254–259. https://doi.org/10.1097/MBC.0b013e3283442ce2
Dahm A, Osterud B, Hjeltnes N, Sandset PM, Iversen PO (2006) Opposite circadian rhythms in melatonin and tissue factor pathway inhibitor type 1: does daylight affect coagulation? J Thromb Haemost 4(8):1840–1842. https://doi.org/10.1111/j.1538-7836.2006.02048.x
Aydogan S, Yerer MB, Goktas A (2006) Melatonin and nitric oxide. J Endocrinol Invest 29(3):281–287. https://doi.org/10.1007/bf03345555
Del Zar MM, Martinuzzo M, Falcón C, Cardinali DP, Carreras LO, Vacas MI (1990) Inhibition of human platelet aggregation and thromboxane-B2 production by melatonin: evidence for a diurnal variation. J Clin Endocrinol Metab 70(1):246–251. https://doi.org/10.1210/jcem-70-1-246
Cardinali DP, Del Zar MM, Vacas MI (1993) The effects of melatonin in human platelets. Acta Physiol Pharmacol Ther Latinoam 43(1–2):1–13
Kornblihtt LI, Finocchiaro L, Molinas FC (1993) Inhibitory effect of melatonin on platelet activation induced by collagen and arachidonic acid. J Pineal Res 14(4):184–191. https://doi.org/10.1111/j.1600-079x.1993.tb00501.x
Sahna E, Parlakpinar H, Turkoz Y, Acet A (2005) Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res 54(5):491–495
Bekyarova G, Tancheva S, Hristova M (2010) The effects of melatonin on burn-induced inflammatory responses and coagulation disorders in rats. Methods Find Exp Clin Pharmacol 32(5):299–303. https://doi.org/10.1358/mf.2010.32.5.1437717
Tunali T, Sener G, Yarat A, Emekli N (2005) Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci 76(11):1259–1265. https://doi.org/10.1016/j.lfs.2004.08.024
Otamas A, Grant PJ, Ajjan RA (2020) Diabetes and atherothrombosis: the circadian rhythm and role of melatonin in vascular protection. Diab Vasc Dis Res 17(3):1
Patel R, Parmar N, Pramanik Palit S, Rathwa N, Ramachandran AV, Begum R (2022) Diabetes mellitus and melatonin: where are we? Biochimie 202:2–14. https://doi.org/10.1016/j.biochi.2022.01.001
Hikichi T, Tateda N, Miura T (2011) Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol. https://doi.org/10.2147/opth.s19559
Peerschke EI, Castellone DD, Stroobants AK, Francis J (2014) Reference range determination for whole-blood platelet aggregation using the Multiplate analyzer. Am J Clin Pathol 142(5):647–656. https://doi.org/10.1309/ajcpp43seycbjlhj
Hsing AW, Meyer TE, Niwa S, Quraishi SM, Chu LW (2010) Measuring serum melatonin in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 19(4):932–937. https://doi.org/10.1158/1055-9965.Epi-10-0004
Carazo A, Hrubša M, Konečný L, Skořepa P, Paclíková M, Musil F et al (2022) Sex-related differences in platelet aggregation: a literature review supplemented with local data from a group of generally healthy individuals. Semin Thromb Hemost (EFirst). https://doi.org/10.1055/s-0042-1756703
Friede KA, Infeld MM, Tan RS, Knickerbocker HJ, Myers RA, Dubois LG et al (2020) Influence of sex on platelet reactivity in response to aspirin. J Am Heart Assoc. https://doi.org/10.1161/jaha.119.014726
Le Blanc J, Lordkipanidzé M (2019) Platelet function in aging. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2019.00109
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351(2):152–166. https://doi.org/10.1016/j.mce.2012.01.004
Hajam YA, Rai S (2020) Melatonin supplementation revives diabetic induced biochemical, histological and hematological impairments in rats. Heliyon 6(4):e03770. https://doi.org/10.1016/j.heliyon.2020.e03770
Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B et al (2018) Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev 39(5):760–802. https://doi.org/10.1210/er.2018-00064
Paterniti I, Campolo M, Cordaro M, Impellizzeri D, Siracusa R, Crupi R et al (2017) PPAR-α modulates the anti-inflammatory effect of melatonin in the secondary events of spinal cord injury. Mol Neurobiol 54(8):5973–5987. https://doi.org/10.1007/s12035-016-0131-9
Shao G, Tian Y, Wang H, Liu F, Xie G (2015) Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int Immunopharmacol 29(2):263–268. https://doi.org/10.1016/j.intimp.2015.11.011
Rhee YH, Ahn JC (2016) Melatonin attenuated adipogenesis through reduction of the CCAAT/enhancer binding protein beta by regulating the glycogen synthase 3 beta in human mesenchymal stem cells. J Physiol Biochem 72(2):145–155. https://doi.org/10.1007/s13105-015-0463-3
Muñoz-Gutiérrez C, Sepúlveda C, Caballero J, Palomo I, Fuentes E (2017) Study of the interactions between edaglitazone and ciglitazone with PPARγ and their antiplatelet profile. Life Sci 186:59–65. https://doi.org/10.1016/j.lfs.2017.07.031
Unsworth AJ, Kriek N, Bye AP, Naran K, Sage T, Flora GD et al (2017) PPARγ agonists negatively regulate αIIbβ3 integrin outside-in signaling and platelet function through up-regulation of protein kinase A activity. J Thromb Haemost 15(2):356–369. https://doi.org/10.1111/jth.13578
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S et al (2017) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J Pineal Res. https://doi.org/10.1111/jpi.12438
Wang Q, Imam MU, Yida Z, Wang F (2017) Peroxisome proliferator-activated receptor gamma (PPARγ) as a target for concurrent management of diabetes and obesity-related cancer. Curr Pharm Des 23(25):3677–3688. https://doi.org/10.2174/1381612823666170704125104
Dhananjay G, Averi AL, Navjot M, Mina P, Thomas LJ, Jack LL (2013) Peroxisome proliferator-activated receptor γ (PPARγ) and its target genes are downstream effectors of foxo1 protein in islet β-cells: Mechanism of β-cell compensation and failure* *This work was supported, in whole or in part, by National Institutes of Health Grant DK56818 (to J. L. L.). This work was also supported by the American Diabetes Association (to M. P.). J Biol Chem 288(35):25440–25449. https://doi.org/10.1074/jbc.M113.486852
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M et al (2016) Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 374(14):1321–1331. https://doi.org/10.1056/NEJMoa1506930
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356(24):2457–2471. https://doi.org/10.1056/NEJMoa072761
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P et al (1987) Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317(20):1237–1245. https://doi.org/10.1056/nejm198711123172001
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB et al (1999) Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med 341(6):410–418. https://doi.org/10.1056/nejm199908053410604
Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR et al (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366(9500):1849–1861. https://doi.org/10.1016/s0140-6736(05)67667-2
Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P et al (2010) Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362(17):1563–1574. https://doi.org/10.1056/NEJMoa1001282
Acknowledgements
We would like to thank our collaborators at Comenius University Science Park, Bratislava, Slovakia for providing laboratory equipment; to Assoc. Prof. Eric Eisenstein for valuable help with language editing and proofreading the manuscript and Ivan Varga, MD, PhD for lending Multiplate® Analyzer for this research.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This study was supported by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic grant (VEGA 1/0563/21).
Author information
Authors and Affiliations
Contributions
All authors have given final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Conceptualization: AB, VL, KD, RM, VM, KP; Data curation: AB, BB, NJ; Formal analysis and investigation: IB, IV, AB, KD, LL, PS, MK, KD; Methodology: BB, NJ, MZ, VM; Project administration: MK, KP; Supervision: MZ, AB; Validation and visualisation: NJ, BB, AB; Writing—original draft: AB, VL, IB, BB; Writing—review and editing: IV, BB, NJ, RM, MK, KP, KD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The research was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the National Cardiovascular Institute, Bratislava, Slovakia.
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Böhm, A., Lauko, V., Dostalova, K. et al. In-vitro antiplatelet effect of melatonin in healthy individuals and patients with type 2 diabetes mellitus. J Endocrinol Invest 46, 2493–2500 (2023). https://doi.org/10.1007/s40618-023-02102-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02102-7