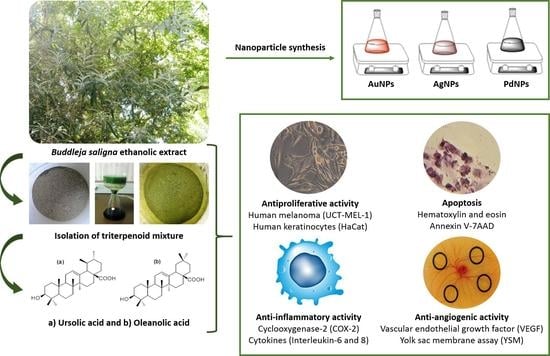
Antiproliferative Activity of Buddleja saligna (Willd.) against Melanoma and In Vivo Modulation of Angiogenesis
Abstract
:1. Introduction
2. Results
2.1. Antiproliferative Activity and Bio-Assay Guided Fractionation
2.2. Apoptosis Detection-Microscopy
2.3. Cyclooxygenase-2 Inhibition
2.4. Cytokine Inhibition
2.5. VEGF Inhibition
2.6. Ex Ovo Yolk Sac Membrane Assay (YSM)
2.7. Nanoparticle Synthesis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Fertilized Eggs
4.4. Preparation of Plant Extract
4.5. Liquid-Liquid Partitioning
4.6. Bioassay-Guided Fractionation
4.7. LC-MS Analysis
4.8. Antiproliferative Activity
4.9. Cell Morphology-Light Microscopy (Hematoxylin and Eosin Staining)
4.10. Cyclooxygenase-2 Inhibition
4.11. Quantification of Human Inflammatory Cytokines
4.12. Quantification of In Vitro VEGF
4.13. Ex Ovo YSM
4.14. Synthesis of Nanoparticles (Gold (Au), Palladium (Pd) and Silver (Ag)
4.15. Characterization of Nanoparticles
4.16. In Vitro Stability of Nanoparticles
4.17. Total Phenolic Content of Nanoparticle Solutions
4.18. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Skin Cancer Foundation Skin Cancer Facts & Statistics. Available online: https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/ (accessed on 7 January 2021).
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global Incidence and Mortality of Skin Cancer by Histological Subtype and Its Relationship with the Human Development Index (HDI); an Ecology Study in 2018. World Cancer Res. J. 2019, 6, e1265. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmett, M.S.; Dewing, D.; Pritchard-Jones, R.O. Angiogenesis and Melanoma-From Basic Science to Clinical Trials. Am. J. Cancer Res. 2011, 1, 868. [Google Scholar]
- Hubler, W.R.; Wolf, J.E. Melanoma.Tumor Angiogenesis and Human Neoplasia. Cancer 1976, 38, 187–192. [Google Scholar] [CrossRef]
- Warren, B.; Shubik, P. The Growth of the Blood Supply to Melanoma Transplants in the Hamster Cheek Pouch-PubMed. Lab. Investig. 1966, 15, 464–478. [Google Scholar] [PubMed]
- Mahabeleshwar, G.H.; Byzova, T.V. Angiogenesis in Melanoma. Semin. Oncol. 2007, 34, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Bar-Eli, M. Role of Interleukin-8 in Tumor Growth and Metastasis of Human Melanoma. Pathobiology 1999, 67, 12–18. [Google Scholar] [CrossRef]
- Nürnberg, W.; Tobias, D.; Otto, F.; Henz, B.M.; Schadendorf, D. Expression of Interleukin-8 Detected by in Situ Hybridization Correlates with Worse Prognosis in Primary Cutaneous Melanoma. J. Pathol. 1999, 189, 546–551. [Google Scholar] [CrossRef]
- Ugurel, S.; Rappl, G.; Tilgen, W.; Reinhold, U. Increased Serum Concentration of Angiogenic Factors in Malignant Melanoma Patients Correlates with Tumor Progression and Survival. J. Clin. Oncol. 2001, 19, 577–583. [Google Scholar] [CrossRef]
- Mahler, D.A.; Huang, S.; Tabrizi, M.; Bell, G.M. Efficacy and Safety of a Monoclonal Antibody Recognizing Lnterleukin-8 in COPD: A Pilot Study. Chest 2004, 126, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Naugler, W.E.; Karin, M. The Wolf in Sheep’s Clothing: The Role of Interleukin-6 in Immunity, Inflammation and Cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and Melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Pinzi, C.; Spallanzani, A.; Berti, E.; Chiarugi, A.; Mazzoli, S.; Fabiani, M.; Vallecchi, C.; Herlyn, M. Immunohistochemical Evidence of Cytokine Networks during Progression of Human Melanocytic Lesions. Int. J. Cancer 1999, 84, 160–168. [Google Scholar] [CrossRef]
- Goulet, A.; Einsphar, J.; Alberts, D.; Beas, A.; Burk, C.; Bhattacharyya, A.; Bangert, J.; Harmon, J.; Fujiwara, H.; Koki, A.; et al. Analysis of Cyclooxygenase 2 (COX-2) Expression during Malignant Melanoma Progression-PubMed. Cancer Biol. Ther. 2003, 2, 713–718. [Google Scholar] [CrossRef]
- Wu, W.K.K.; Yiu Sung, J.J.; Lee, C.W.; Yu, J.; Cho, C.H. Cyclooxygenase-2 in Tumorigenesis of Gastrointestinal Cancers: An Update on the Molecular Mechanisms. Cancer Lett. 2010, 295, 7–16. [Google Scholar] [CrossRef]
- National Cancer Institute Bevacizumab. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/bevacizumab (accessed on 7 January 2021).
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R. Bombesin Functionalized Gold Nanoparticles Show in Vitro and in Vivo Cancer Receptor Specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef] [Green Version]
- Thipe, V.C.; Amiri, K.P.; Bloebaum, P.; Raphael, A.K.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of Resveratrol-Conjugated Gold Nanoparticles: Interrelationship of Increased Resveratrol Corona on Anti-Tumor Efficacy against Breast, Pancreatic and Prostate Cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef] [Green Version]
- Tangthong, T.; Piroonpan, T.; Thipe, V.C.; Khoobchandani, M.; Katti, K.; Katti, K.V.; Pasanphan, W. Water-Soluble Chitosan Conjugated DOTA-Bombesin Peptide Capped Gold Nanoparticles as a Targeted Therapeutic Agent for Prostate Cancer. Nanotechnol. Sci. Appl. 2021, 14, 69–89. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Bloebaum, P.L.R.; Katti, K.V. Targeted Phytochemical-Conjugated Gold Nanoparticles in Cancer Treatment. In Biotechnology Products in Everyday Life; Springer International Publishing: Cham, Switzerland, 2019; ISBN 9783319923994. [Google Scholar]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Dhurvas Mohandoss, D.K.; Darshakumar, R.D.; Joshi, C.M.; Katti, V.K. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine-Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020, 15, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P. V Counteracting the Effect of Leukemia Exosomes by Antiangiogenic Gold Nanoparticles. Int. J. Nanomed. 2019, 14, 6843–6854. [Google Scholar] [CrossRef] [Green Version]
- Baharara, J.; Namvar, F.; Mousavi, M.; Ramezani, T.; Mohamad, R. Anti-Angiogenesis Effect of Biogenic Silver Nanoparticles Synthesized Using Saliva Officinalis on Chick Chorioalantoic Membrane (CAM). Molecules 2014, 19, 13498–13508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhang, W.; Desikan Seshadri, V.D.; Cao, G. Synthesis and Characterization of Gold Nanoparticles from Marsdenia Tenacissima and Its Anticancer Activity of Liver Cancer HepG2 Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a First-in-Class Radioenhancer Hafnium Oxide Nanoparticle, plus Radiotherapy versus Radiotherapy Alone in Patients with Locally Advanced Soft-Tissue Sarcoma (Act.In.Sarc): A Multicentre, Phase 2–3, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2021, 12, 500–525. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.; Cragg, G. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Howes, M.-J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.-E.; Leaman, D.J.; et al. Molecules from Nature: Reconciling Biodiversity Conservation and Global Healthcare Imperatives for Sustainable Use of Medicinal Plants and Fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Koh, H.K.; Geller, A.C.; Miller, D.R.; Grossbart, T.A.; Lew, R.A. Prevention and Early Detection Strategies for Melanoma and Skin Cancer. Current Status. Arch. Dermatol. 1996, 132, 436–443. [Google Scholar] [CrossRef]
- Aubrey, A. Buddleja Saligna|PlantZAfrica. Available online: http://pza.sanbi.org/buddleja-saligna (accessed on 30 October 2021).
- Su, M.; Huang, J.; Liu, S.; Xiao, Y.; Qin, X.; Liu, J.; Pi, C.; Luo, T.; Li, J.; Chen, X.; et al. The Anti-Angiogenic Effect and Novel Mechanisms of Action of Combretastatin A-4. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bamuamba, K.; Gammon, D.; Meyers, P.; Dijoux-Franca, M.; Scott, G. Anti-Mycobacterial Activity of Five Plant Species Used as Traditional Medicines in the Western Cape Province (South Africa). J. Ethnopharmacol. 2008, 117, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Chukwujekwu, J.; Amoo, S.; de Kock, C.; Smith, P.; Van Staden, J. Antiplasmodial, Acetylcholinesterase and Alpha-Glucosidase Inhibitory and Cytotoxicity Properties of Buddleja Saligna. S. Afr. J. Bot. 2014, 94, 6–8. [Google Scholar] [CrossRef]
- Singh, A.; Venugopala, K.; Khedr, M.; Pillay, M.; Nwaeze, K.; Coovadia, Y.; Shode, F.; Odhav, B. Antimycobacterial, Docking and Molecular Dynamic Studies of Pentacyclic Triterpenes from Buddleja Saligna Leaves. J. Biomol. Struct. Dyn. 2017, 35, 2654–2664. [Google Scholar] [CrossRef]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of Ursolic and Oleanolic on SK-MEL-2 Melanoma Cells: In Vitro and in Vivo Assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, M.; Rabe, S.; Balali-Mood, M.; Karimi, G.; Tabasi, N.; Riahi-Zanjani, B. Ursolic Acid Induced Apoptotic Cell Death Following Activation of Caspases in Isolated Human Melanoma Cells. Cell Biol. Int. 2015, 39, 230–236. [Google Scholar] [CrossRef]
- Oprean, C.; Ivan, A.; Bojin, F.; Cristea, M.; Soica, C.; Drăghia, L.; Caunii, A.; Paunescu, V.; Tatu, C. Selective in Vitro Anti-Melanoma Activity of Ursolic and Oleanolic Acids. Toxicol. Mech. Methods 2018, 28, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bishayee, K.; Khuda-Bukhsh, A. Oleanolic Acid Isolated from Ethanolic Extract of Phytolacca Decandra Induces Apoptosis in A375 Skin Melanoma Cells: Drug-DNA Interaction and Signaling Cascade. J. Integr. Med. 2014, 12, 102–114. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Rengasamy, K.R.R.; Kock, C.A.d.; Smith, P.J.; Slavětínská, L.P.; van Staden, J. Alpha-Glucosidase Inhibitory and Antiplasmodial Properties of Terpenoids from the Leaves of Buddleja saligna Willd. J. Enzym. Inhib. Med. Chem. 2015, 31, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Pratheeshkumar, P.; Kuttan, G. Oleanolic Acid Induces Apoptosis by Modulating P53, Bax, Bcl-2 and Caspase-3 Gene Expression and Regulates the Activation of Transcription Factors and Cytokine Profile in B16F. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 21–31. [Google Scholar] [CrossRef]
- Fuliaş, A.; Ledeţi, I.; Vlase, G.; Vlase, T.; Şoica, C.; Dehelean, C.; Oprean, C.; Bojin, F.; Şuta, L.-M.; Bercean, V.; et al. Thermal Degradation, Kinetic Analysis, and Apoptosis Induction in Human Melanoma for Oleanolic and Ursolic Acids. J. Therm. Anal. Calorim. 2015, 125, 759–768. [Google Scholar] [CrossRef]
- Harmand, P.; Duval, R.; Delage, C.; Simon, A. Ursolic Acid Induces Apoptosis through Mitochondrial Intrinsic Pathway and Caspase-3 Activation in M4Beu Melanoma Cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.; Kuttan, G. Ursolic Acid Induces Apoptosis by Activating P53 and Caspase-3 Gene Expressions and Suppressing NF-KappaB Mediated Activation of Bcl-2 in B16F-10 Melanoma Cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Ringbom, T.; Segura, L.; Noreen, Y.; Perera, P.; Bohlin, L. Ursolic Acid from Plantago Major, a Selective Inhibitor of Cyclooxygenase-2 Catalyzed Prostaglandin Biosynthesis. J. Nat. Prod. 1998, 61, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Dannenberg, A.J. Cyclooxygenase 2: A Molecular Target for Cancer Prevention and Treatment. Trends Pharmacol. Sci. 2003, 24, 96–102. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Han, S.; Kim, H. Ursolic Acid Isolated from Guava Leaves Inhibits Inflammatory Mediators and Reactive Oxygen Species in LPS-Stimulated Macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 228–235. [Google Scholar] [CrossRef]
- Li, M.; Han, Z.; Bei, W.; Rong, X.; Guo, J.; Hu, X. Oleanolic Acid Attenuates Insulin Resistance via NF-ΚB to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells. Evid. Based. Complement. Alternat. Med. 2015, 2015, 643102. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Han, S.; Sung, H.; Park, S.; Kang, M.; Han, S.; Kang, Y. Blockade of Visfatin Induction by Oleanolic Acid via Disturbing IL-6-TRAF6-NF-ΚB Signaling of Adipocytes. Exp. Biol. Med. 2014, 239, 284–292. [Google Scholar] [CrossRef]
- Lee, C.; Wu, S.; Chen, J.; Li, C.; Lo, H.; Cheng, W.; Lin, J.; Chang, Y.; Hsiang, C.; Ho, T. Eriobotrya Japonica Leaf and Its Triterpenes Inhibited Lipopolysaccharide-Induced Cytokines and Inducible Enzyme Production via the Nuclear Factor-KappaB Signaling Pathway in Lung Epithelial Cells. Am. J. Chin. Med. 2008, 36, 1185–1198. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, R.; Lou, B.; Cheng, K.; Xiong, J.; Hu, J. Chemical Constituents from Melastoma Dodecandrum and Their Inhibitory Activity on Interleukin-8 Production in HT-29 Cells. Nat. Prod. Res. 2014, 28, 1383–1387. [Google Scholar] [CrossRef]
- Kanjoormana, M.; Kuttan, G. Antiangiogenic Activity of Ursolic Acid. Integr. Cancer Ther. 2010, 9, 224–235. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, J.; Jeon, J.; Kim, K.-J.; Yun, J.-H.; Jeong, H.-S.; Lee, E.H.; Koh, Y.J.; Cho, C.-H. Oleanolic Acids Inhibit Vascular Endothelial Growth Factor Receptor 2 Signaling in Endothelial Cells: Implication for Anti-Angiogenic Therapy. Mol. Cells 2018, 41, 780. [Google Scholar] [CrossRef]
- Cárdenas, C.; Quesada, A.R.; Medina, M.Á. Effects of Ursolic Acid on Different Steps of the Angiogenic Process. Biochem. Biophys. Res. Commun. 2004, 320, 402–408. [Google Scholar] [CrossRef]
- Sohn, K.; Lee, H.; Chung, H.; Young, H.; Yi, S.; Kim, K. Anti-Angiogenic Activity of Triterpene Acids. Cancer Lett. 1995, 94, 213–218. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green Synthesis of Silver Nanoparticles from Marigold Flower and Its Synergistic Antimicrobial Potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Reducing Agents. Int. J. Nanomed. 2014, 9, 4021. [Google Scholar] [CrossRef] [Green Version]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green Synthesis of Palladium Nanoparticles Mediated by Black Tea Leaves (Camellia sinensis) Extract: Catalytic Activity in the Reduction of 4-Nitrophenol and Suzuki-Miyaura Coupling Reaction under Ligand-Free Conditions. J. Colloid Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef]
- Twilley, D.; Moodley, D.; Rolfes, H.; Moodley, I.; McGaw, L.J.; Madikizela, B.; Summers, B.; Raaff, L.A.; Lategan, M.; Kgatuke, L.; et al. Ethanolic Extracts of South African Plants, Buddleja saligna Willd. and Helichrysum odoratissimum (L.) Sweet, as Multifunctional Ingredients in Sunscreen Formulations. S. Afr. J. Bot. 2021, 137, 171–182. [Google Scholar] [CrossRef]
- Lall, N.; Henley-Smith, C.J.; Canha, M.N.D.; Oosthuizen, C.B.; Berrington, D. Viability Reagent, PrestoBlue, in Comparison with Other Available Reagents, Utilized in Cytotoxicity and Antimicrobial Assays. Int. J. Microbiol. 2013, 2013, 420601. [Google Scholar] [CrossRef] [Green Version]
- Reininger, E.A.; Bauer, R. Prostaglandin-H-Synthase (PGHS)-1 and -2 Microtiter Assays for the Testing of Herbal Drugs and in Vitro Inhibition of PGHS-Isoenzyms by Polyunsaturated Fatty Acids from Platycodi Radix. Phytomedicine 2006, 13, 164–169. [Google Scholar] [CrossRef]
- Twilley, D.; Langhansová, L.; Palaniswamy, D.; Lall, N. Evaluation of Traditionally Used Medicinal Plants for Anticancer, Antioxidant, Anti-Inflammatory and Anti-Viral (HPV-1) Activity. S. Afr. J. Bot. 2017, 112, 494–500. [Google Scholar] [CrossRef]
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A.-A. The Effects of Resveratrol on the Expression of VEGF, TGF-β, and MMP-9 in Endometrial Stromal Cells of Women with Endometriosis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Nihad, A.S.; Deshpande, R.; Kale, V.P.; Bhonde, R.R.; Datar, S.P. Establishment of an in Ovo Chick Embryo Yolk Sac Membrane (YSM) Assay for Pilot Screening of Potential Angiogenic and Anti-Angiogenic Agents. Cell Biol. Int. 2018, 42, 1474–1483. [Google Scholar] [CrossRef]
- Datar, S.; Shah, R.; Talele, G.; Bhonde, R. In Vivo Screening of Potential Anti-Angiogenic Homeopathic Medicines Using Chick Embryo YSM Model. Cell Cell. Life Sci. J. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Kuhnen, S.; Lemos, P.M.M.; Campestrini, L.H.; Ogliari, J.B.; Dias, P.F.; Maraschin, M. Antiangiogenic Properties of Carotenoids: A Potential Role of Maize as Functional Food. J. Funct. Foods 2009, 1, 284–290. [Google Scholar] [CrossRef]
- Wang, H.; Cheng-yu, L.; Wang, X.; Bao, Y.; Meng, X.; Yin, W.; Yu-xin, L. Chick Yolk Sac Membrane Assay: A Novel Angiogenesis Model. J. Biol. Res. 2007, 7, 93–97. [Google Scholar]
- Zhou, Q.; Qi, C.L.; Li, Y.; He, X.D.; Li, J.C.; Zhang, Q.Q.; Tian, L.; Zhang, M.; Han, Z.; Wang, H.; et al. A Novel Four-Step System for Screening Angiogenesis Inhibitors. Mol. Med. Rep. 2013, 8, 1734–1740. [Google Scholar] [CrossRef] [Green Version]
- Belleri, M.; Ribatti, D.; Nicoli, S.; Cotelli, F.; Forti, L.; Vannini, V.; Stivala, L.A.; Presta, M. Antiangiogenic and Vascular-Targeting Activity of the Microtubule-Destabilizing Trans-Resveratrol Derivative 3,5,4′-Trimethoxystilbene. Mol. Pharmacol. 2005, 67, 1451–1459. [Google Scholar] [CrossRef]
- Belleri, M.; Ribatti, D.; Savio, M.; Stivala, L.A.; Forti, L.; Tanghetti, E.; Alessi, P.; Coltrini, D.; Bugatti, A.; Mitola, S.; et al. Alphavbeta3 Integrin-Dependent Antiangiogenic Activity of Resveratrol Stereoisomers. Mol. Cancer Ther. 2008, 7, 3761–3770. [Google Scholar] [CrossRef]









| Sample | UCT-MEL-1 Cells | HaCat Cells | Selectivity Index |
|---|---|---|---|
| IC50 ± SD (µg/mL) | |||
| Buddleja saligna ethanolic extract | 33.80 ± 1.02 | 54.38 ± 8.55 | 1.64 |
| Hexane partition | 109.92 ± 20.05 | − 1 | NA 2 |
| Dichloromethane (DCM) partition | 15.72 ± 1.34 | − | NA |
| Water partition | 57.69 ± 5.28 | − | NA |
| M1 fraction of DCM partition | >200 | − | NA |
| M2 fraction of DCM partition | 28.89 ± 3.61 | − | NA |
| M3 fraction of DCM partition | 24.10 ± 3.07 | − | NA |
| M4 fraction of DCM partition | 10.73 ± 3.40 | − | NA |
| DT-BS-01 subfraction of M4 | 5.45 ± 0.19 | 27.59 ± 2.86 | 5.06 |
| Ursolic acid | 2.31 ± 0.54 | 2.32 ± 0.52 | 1.01 |
| Oleanolic acid | 13.08 ± 3.03 | 16.84 ± 1.32 | 1.29 |
| Actinomycin D | 2.59 × 10 3 ± 4.85 × 10−4 | 5.57 × 10−3 ± 2.50 × 10−4 | 1.52 |
| Sample | Hydrodynamic Size (nm) | PDI 1 | Zeta Potential (mV) | TEM Size (nm) | Surface Coating (nm) | Total Phenolic Content (µg/mL GAE 2) | UCT-MEL-1 Cells | HaCat Cells | RAW 264.7 Cells |
|---|---|---|---|---|---|---|---|---|---|
| IC50 ± SD (µg/mL) | |||||||||
| BS 3-AuNPs 4 | 68.87 ± 1.0 | 0.3 | −33.6 ± 1.0 | 16.8 ± 11.7 | 52.1 | 182.7 | 42.72 ± 2.07 | 72.81 ± 4.89 | 17.49 ± 2.15 |
| BS-AgNPs | 42.22 ± 1.0 | 0.5 | −30.1 ± 1.4 | 14.5 ± 7.7 | 27.7 | 292.4 | 16.00 ± 1.92 | 37.49 ± 0.19 | 37.54 ± 0.15 |
| BS-PdNPs | 94.70 ± 1.4 | 0.3 | −16 ± 0.1 | 5.3 ± 2.9 | 89.4 | 165 | 33.74 ± 7.4 | 38.97 ± 0.42 | 74.57 ± 1.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twilley, D.; Thipe, V.C.; Kishore, N.; Bloebaum, P.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R.; Selepe, M.A.; Langhansova, L.; Katti, K.; et al. Antiproliferative Activity of Buddleja saligna (Willd.) against Melanoma and In Vivo Modulation of Angiogenesis. Pharmaceuticals 2022, 15, 1497. https://doi.org/10.3390/ph15121497
Twilley D, Thipe VC, Kishore N, Bloebaum P, Roma-Rodrigues C, Baptista PV, Fernandes AR, Selepe MA, Langhansova L, Katti K, et al. Antiproliferative Activity of Buddleja saligna (Willd.) against Melanoma and In Vivo Modulation of Angiogenesis. Pharmaceuticals. 2022; 15(12):1497. https://doi.org/10.3390/ph15121497
Chicago/Turabian StyleTwilley, Danielle, Velaphi C. Thipe, Navneet Kishore, Pierce Bloebaum, Catarina Roma-Rodrigues, Pedro V. Baptista, Alexandra R. Fernandes, Mamoalosi A. Selepe, Lenka Langhansova, Kattesh Katti, and et al. 2022. "Antiproliferative Activity of Buddleja saligna (Willd.) against Melanoma and In Vivo Modulation of Angiogenesis" Pharmaceuticals 15, no. 12: 1497. https://doi.org/10.3390/ph15121497