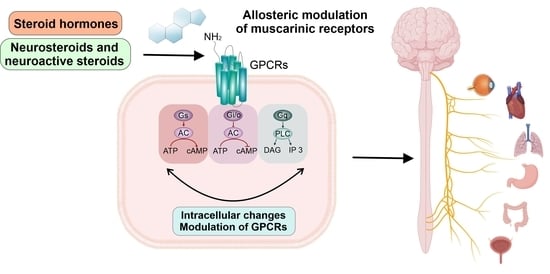
Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids
Abstract
:1. Introduction
2. Cholesterol, Neurosteroids and Neuroactive Steroids
2.1. Cholesterol
2.2. NSs, NASs–Functions and Their Genomic and Non-Genomic Effects
3. Muscarinic Receptors
4. Direct Effects of Steroids on mAChRs
4.1. Direct Effects of Cholesterol
4.2. Non-Genomic Effects of NSs and NASs on mAChRs
5. Conclusions
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonner, T.I.; Buckley, N.J.; Young, A.C.; Brann, M.R. Identification of a Family of Muscarinic Acetylcholine Receptor Genes. Science 1987, 237, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Caulfield, M.P. Muscarinic Receptors—Characterization, Coupling and Function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar] [CrossRef]
- Eglen, R.M. Overview of Muscarinic Receptor Subtypes. In Handb Exp Pharmacol; Fryer, A.D., Arthur Christopoulos, N.N.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–28. ISBN 0171-2004. [Google Scholar]
- Scarr, E. Muscarinic Receptors: Their Roles in Disorders of the Central Nervous System and Potential as Therapeutic Targets. CNS Neurosci. Ther. 2012, 18, 369–379. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.-E.; Buccafusco, J.J.; Chapple, C.; De Groat, W.C.; Fryer, A.; Kay, G.; Laties, A.; Nathanson, N.; Pasricha, P.J.; et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. J. Cereb. Blood Flow Metab. 2006, 148, 565–578. [Google Scholar] [CrossRef] [Green Version]
- Gosens, R.; Zaagsma, J.; Meurs, H.; Halayko, A.J. Muscarinic Receptor Signaling in the Pathophysiology of Asthma and COPD. Respir. Res. 2006, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Gautam, D.; Han, S.J.; Duttaroy, A.; Mears, D.; Hamdan, F.F.; Li, J.H.; Cui, Y.; Jeon, J.; Wess, J. Role of the M3 Muscarinic Acetylcholine Receptor in β-Cell Function and Glucose Homeostasis. Diabetes, Obes. Metab. 2007, 9, 158–169. [Google Scholar] [CrossRef]
- Krejcí, A.; Tucek, S. Changes of Cooperativity between N-Methylscopolamine and Allosteric Modulators Alcuronium and Gallamine Induced by Mutations of External Loops of Muscarinic M(3) Receptors. Mol. Pharmacol. 2001, 60, 761–767. [Google Scholar]
- Mysliveček, J.; Říčný, J.; Kolář, F.; Tuček, S. The Effects of Hydrocortisone on Rat Heart Muscarinic and Adrenergic A1, Β1 and Β2 Receptors, Propranolol-Resistant Binding Sites and on Some Subsequent Steps in Intracellular Signalling. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003, 368, 366–376. [Google Scholar] [CrossRef]
- Jakubik, J.; El-Fakahany, E.E. Current Advances in Allosteric Modulation of Muscarinic Receptors. Biomolecules 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Doruker, P.; Kaynak, B.; Zhang, S.; Krieger, J.; Li, H.; Bahar, I. Intrinsic Dynamics Is Evolutionarily Optimized to Enable Allosteric Behavior. Curr. Opin. Struct. Biol. 2020, 62, 14–21. [Google Scholar] [CrossRef]
- Clark, A.L.; Mitchelson, F. The Inhibitory Effect of Gallamine on Muscarinic Receptors. Br. J. Pharmacol. 1976, 58, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Stockton, J.M.; Birdsall, N.J.; Burgen, A.S.; Hulme, E.C. Modification of the Binding Properties of Muscarinic Receptors by Gallamine. Mol. Pharmacol. 1983, 23, 551–557. [Google Scholar] [PubMed]
- Nedoma, J.; Dorofeeva, N.A.; Tuček, S.; Shelkovnikov, S.A.; Danilov, A.F. Interaction of the Neuromuscular Blocking Drugs Alcuronium, Decamethonium, Gallamine, Pancuronium, Ritebronium, Tercuronium and d-Tubocurarine with Muscarinic Acetylcholine Receptors in the Heart and Ileum. Naunyn Schmiedebergs Arch. Pharmacol. 1985, 329, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Lazareno, S.; Dolezal, V.; Popham, A.; Birdsall, N.J.M. Thiochrome Enhances Acetylcholine Affinity at Muscarinic M4 Receptors: Receptor Subtype Selectivity via Cooperativity Rather than Affinity. Mol. Pharmacol. 2004, 65, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Waelbroeck, M.; Robberecht, P.; De Neef, P.; Christophe, J. Effects of Verapamil on the Binding Properties of Rat Heart Muscarinic Receptors: Evidence for an Allosteric Site. Biochem. Biophys. Res. Commun. 1984, 121, 340–345. [Google Scholar] [CrossRef]
- Proska, J.; Tucek, S. Competition between Positive and Negative Allosteric Effectors on Muscarinic Receptors. Mol. Pharmacol. 1995, 48, 696–702. [Google Scholar]
- Proška, J.; Tuček, S. Positive Allosteric Action of Eburnamonine on Cardiac Muscarinic Acetylcholine Receptors. Eur. J. Pharmacol. 1996, 305, 201–205. [Google Scholar] [CrossRef]
- Dong, G.Z.; Kameyama, K.; Rinken, A.; Haga, T. Ligand Binding Properties of Muscarinic Acetylcholine Receptor Subtypes (M1-M5) Expressed in Baculovirus-Infected Insect Cells. J. Pharmacol. Exp. Ther. 1995, 274, 378–384. [Google Scholar]
- Dong, G.Z.; Haga, T.; Itokawa, H.; Mizobe, F. Allosteric Binding of 9-Methoxy-Alpha-Lapachone and Alcuronium to the Muscarinic Acetylcholine Receptor M2 Subtype. Biomed. Res. 1995, 16, 327–335. [Google Scholar] [CrossRef]
- Birdsall, N.J.M.; Lazareno, S. Allosterism at Muscarinic Receptors: Ligands and Mechanisms. Mini Rev. Med. Chem. 2005, 5, 523–543. [Google Scholar] [CrossRef]
- Gregory, K.J.; Sexton, P.M.; Christopoulos, A. Allosteric Modulation of Muscarinic Acetylcholine Receptors. Curr. Neuropharmacol. 2007, 5, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubík, J.; El-Fakahany, E.E. Allosteric Modulation of Muscarinic Acetylcholine Receptors. Pharmaceuticals 2010, 3, 2838–2860. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Seager, M.A.; Seager, M.; Wittmann, M.; Jacobson, M.; Bickel, D.; Burno, M.; Jones, K.; Graufelds, V.K.; Xu, G.; et al. Selective Activation of the M1 Muscarinic Acetylcholine Receptor Achieved by Allosteric Potentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 15950–15955. [Google Scholar] [CrossRef] [PubMed]
- Shirey, J.K.; Brady, A.E.; Jones, P.J.; Davis, A.A.; Bridges, T.M.; Kennedy, J.P.; Jadhav, S.B.; Menon, U.N.; Xiang, Z.; Watson, M.L.; et al. A Selective Allosteric Potentiator of the M1 Muscarinic Acetylcholine Receptor Increases Activity of Medial Prefrontal Cortical Neurons and Restores Impairments in Reversal Learning. J. Neurosci. 2009, 29, 14271–14286. [Google Scholar] [CrossRef] [Green Version]
- Shirey, J.K.; Xiang, Z.; Orton, D.; Brady, A.E.; Johnson, K.A.; Williams, R.; Ayala, J.E.; Rodriguez, A.L.; Wess, J.; Weaver, D.; et al. An Allosteric Potentiator of M4 MAChR Modulates Hippocampal Synaptic Transmission. Nat. Chem. Biol. 2008, 4, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.E.; Jones, C.K.; Bridges, T.M.; Kennedy, J.P.; Thompson, A.D.; Heiman, J.U.; Breininger, M.L.; Gentry, P.R.; Yin, H.; Jadhav, S.B.; et al. Centrally Active Allosteric Potentiators of the M4 Muscarinic Acetylcholine Receptor Reverse Amphetamine-Induced Hyperlocomotor Activity in Rats. J. Pharmacol. Exp. Ther. 2008, 327, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.Y.; McKinzie, D.L.; Bose, S.; Mitchell, S.N.; Witkin, J.M.; Thompson, R.C.; Christopoulos, A.; Lazareno, S.; Birdsall, N.J.M.; Bymaster, F.P.; et al. Allosteric Modulation of the Muscarinic M4 Receptor as an Approach to Treating Schizophrenia. Proc. Natl. Acad. Sci. USA 2008, 105, 10978–10983. [Google Scholar] [CrossRef] [Green Version]
- Michal, P.; Rudajev, V.; El-Fakahany, E.E.; Dolezal, V. Membrane Cholesterol Content Influences Binding Properties of Muscarinic M2 Receptors and Differentially Impacts Activation of Second Messenger Pathways. Eur. J. Pharmacol. 2009, 606, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Michal, P.; El-Fakahany, E.E.; Doležal, V. Changes in Membrane Cholesterol Differentially Influence Preferential and Non-Preferential Signaling of the M1 and M3 Muscarinic Acetylcholine Receptors. Neurochem. Res. 2015, 40, 2068–2077. [Google Scholar] [CrossRef] [Green Version]
- Randáková, A.; Dolejší, E.; Rudajev, V.; Zimčík, P.; Doležal, V.; El-Fakahany, E.E.; Jakubík, J. Role of Membrane Cholesterol in Differential Sensitivity of Muscarinic Receptor Subtypes to Persistently Bound Xanomeline. Neuropharmacology 2018, 133, 129–144. [Google Scholar] [CrossRef]
- Acconcia, F.; Marino, M. Steroid Hormones: Synthesis, Secretion, and Transport. In Principles of Endocrinology and Hormone Action; Belfiore, A., LeRoith, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–31. ISBN 978-3-319-27318-1. [Google Scholar]
- Rupprecht, R.; Holsboer, F. Neuroactive Steroids: Mechanisms of Action and Neuropsychopharmacological Perspectives. Trends Neurosci. 1999, 22, 410–416. [Google Scholar] [CrossRef]
- Do Rego, J.L.; Seong, J.Y.; Burel, D.; Leprince, J.; Luu-The, V.; Tsutsui, K.; Tonon, M.C.; Pelletier, G.; Vaudry, H. Neurosteroid Biosynthesis: Enzymatic Pathways and Neuroendocrine Regulation by Neurotransmitters and Neuropeptides. Front. Neuroendocrinol. 2009, 30, 259–301. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids: Endogenous Role in the Human Brain and Therapeutic Potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef]
- Wilkenfeld, S.R.; Lin, C.; Frigo, D.E. Communication between Genomic and Non-Genomic Signaling Events Coordinate Steroid Hormone Actions. Steroids 2018, 133, 2–7. [Google Scholar] [CrossRef]
- Zhao, X.F. G Protein-Coupled Receptors Function as Cell Membrane Receptors for the Steroid Hormone 20-Hydroxyecdysone. Cell Commun. Signal. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Baulieu, E.; Robel, P. Neurosteroids: A New Brain Function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef]
- Colciago, A.; Bonalume, V.; Melfi, V.; Magnaghi, V. Genomic and Non-Genomic Action of Neurosteroids in the Peripheral Nervous System. Front. Neurosci. 2020, 14, 796. [Google Scholar] [CrossRef]
- Daniel, J.M.; Hulst, J.L.; Lee, C.D. Role of Hippocampal M2 Muscarinic Receptors in the Estrogen-Induced Enhancement of Working Memory. Neuroscience 2005, 132, 57–64. [Google Scholar] [CrossRef]
- Darnaudéry, M.; Koehl, M.; Piazza, P.V.; Le Moal, M.; Mayo, W. Pregnenolone Sulfate Increases Hippocampal Acetylcholine Release and Spatial Recognition. Brain Res. 2000, 852, 173–179. [Google Scholar] [CrossRef]
- Horishita, T.; Minami, K.; Uezono, Y.; Shiraishi, M.; Ogata, J.; Okamoto, T.; Terada, T.; Sata, T. The Effects of the Neurosteroids: Pregnenolone, Progesterone and Dehydroepiandrosterone on Muscarinic Receptor-Induced Responses in Xenopus Oocytes Expressing M1 and M3 Receptors. Naunyn. Schmiedebergs. Arch. Pharmacol. 2005, 371, 221–228. [Google Scholar] [CrossRef]
- Klangkalya, B.; Chan, A. The Effects of Ovarian Hormones on Beta-Adrenergic and Muscarinic Receptors in Rat Heart. Life Sci. 1988, 42, 2307–2314. [Google Scholar] [CrossRef]
- Klangkalya, B.; Chan, A. Structure-Activity Relationships of Steroid Hormones on Muscarinic Receptor Binding. J. Steroid Biochem. 1988, 29, 111–118. [Google Scholar] [CrossRef]
- Klangkalya, B.; Chan, A. Inhibition of Hypothalamic and Pituitary Muscarinic Receptor Binding by Progesterone. Neuroendocrinology 1988, 47, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Dolejší, E.; Szánti-Pintér, E.; Chetverikov, N.; Nelic, D.; Randáková, A.; Doležal, V.; Kudová, E.; Jakubík, J. Neurosteroids and Steroid Hormones Are Allosteric Modulators of Muscarinic Receptors. Neuropharmacology 2021, 199, 108798. [Google Scholar] [CrossRef]
- Dolejší, E.; Chetverikov, N.; Szánti-Pintér, E.; Nelic, D.; Randáková, A.; Doležal, V.; El-Fakahany, E.E.; Kudová, E.; Jakubík, J. Neuroactive Steroids, WIN-Compounds and Cholesterol Share a Common Binding Site on Muscarinic Acetylcholine Receptors. Biochem. Pharmacol. 2021, 192, 114699. [Google Scholar] [CrossRef]
- Fantini, J.; Barrantes, F.J. How Cholesterol Interacts with Membrane Proteins: An Exploration of Cholesterol-Binding Sites Including CRAC, CARC, and Tilted Domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Bandara, A.; Panahi, A.; Pantelopulos, G.A.; Straub, J.E. Exploring the Structure and Stability of Cholesterol Dimer Formation in Multicomponent Lipid Bilayers. J. Comput. Chem. 2017, 38, 1479–1488. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Lei, B.; Morris, D.P.; Smith, M.P.; Schwinn, D.A. Lipid Rafts Constrain Basal A1A-Adrenergic Receptor Signaling by Maintaining Receptor in an Inactive Conformation. Cell. Signal. 2009, 21, 1532–1539. [Google Scholar] [CrossRef]
- Niemelä, P.S.; Ollila, S.; Hyvönen, M.T.; Karttunen, M.; Vattulainen, I. Assessing the Nature of Lipid Raft Membranes. PLoS Comput. Biol. 2007, 3, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Levitan, I.; Fang, Y.; Rosenhouse-Dantsker, A.; Romanenko, V. Cholesterol and Ion Channels. Subcell. Biochem. 2010, 51, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, A.L.; Song, W.; Sansom, M.S.P. Lipid-Dependent Regulation of Ion Channels and G Protein–Coupled Receptors: Insights from Structures and Simulations. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Burger, K.; Fahrenholz, F. A Closer Look at the Cholesterol Sensor. Trends Biochem. Sci. 2002, 27, 596–599. [Google Scholar] [CrossRef]
- Hanson, M.A.; Cherezov, V.; Griffith, M.T.; Roth, C.B.; Jaakola, V.-P.; Chien, E.Y.T.; Velasquez, J.; Kuhn, P.; Stevens, R.C. A Specific Cholesterol Binding Site Is Established by the 2.8 A Structure of the Human Beta2-Adrenergic Receptor. Structure 2008, 16, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Paila, Y.D.; Tiwari, S.; Chattopadhyay, A. Are Specific Nonannular Cholesterol Binding Sites Present in G-Protein Coupled Receptors? Biochim. Biophys. Acta Biomembr. 2009, 1788, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimpl, G. Interaction of G Protein Coupled Receptors and Cholesterol. Chem. Phys. Lipids 2016, 199, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Chattopadhyay, A. Cholesterol Interaction Motifs in G Protein-Coupled Receptors: Slippery Hot Spots? Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1481. [Google Scholar] [CrossRef]
- Reddy, D.S.; Estes, W.A. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol. Sci. 2016, 37, 543–561. [Google Scholar] [CrossRef] [Green Version]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; González, S.L. Neuroactive Steroids, Nociception and Neuropathic Pain: A Flashback to Go Forward. Steroids 2016, 110, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Joksimovic, S.L.; Covey, D.F.; Jevtovic-Todorovic, V.; Todorovic, S.M. Neurosteroids in Pain Management: A New Perspective on an Old Player. Front. Pharmacol. 2018, 9, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, L.; Taleb, O.; Patte-Mensah, C.; Mensah-Nyagan, A.-G. Neurosteroids and Neuropathic Pain Management: Basic Evidence and Therapeutic Perspectives. Front. Neuroendocrinol. 2019, 55, 100795. [Google Scholar] [CrossRef] [PubMed]
- González, S.L.; Meyer, L.; Raggio, M.C.; Taleb, O.; Coronel, M.F.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Allopregnanolone and Progesterone in Experimental Neuropathic Pain: Former and New Insights with a Translational Perspective. Cell. Mol. Neurobiol. 2019, 39, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, K.K.; Piskorska, B.; Banach, M.; Czuczwar, S.J. Neuroprotective Actions of Neurosteroids. Front. Endocrinol. 2011, 2, 50. [Google Scholar] [CrossRef] [Green Version]
- Mendell, A.L.; MacLusky, N.J. Neurosteroid Metabolites of Gonadal Steroid Hormones in Neuroprotection: Implications for Sex Differences in Neurodegenerative Disease. Front. Mol. Neurosci. 2018, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as Regulators of Neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Kudova, E. Rapid Effects of Neurosteroids on Neuronal Plasticity and Their Physiological and Pathological Implications. Neurosci. Lett. 2021, 750, 135771. [Google Scholar] [CrossRef]
- Klinge, C.M. Steroid Hormone Receptors and Signal Transduction Processes. In Principles of Endocrinology and Hormone Action; Springer: Cham, Switzerland, 2018; pp. 187–232. [Google Scholar]
- Reddy, D.S. Catamenial Epilepsy: Discovery of an Extrasynaptic Molecular Mechanism for Targeted Therapy. Front. Cell. Neurosci. 2016, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Baulieu, E.E. Neurosteroids: A Novel Function of the Brain. Psychoneuroendocrinology 1998, 23, 963–987. [Google Scholar] [CrossRef]
- Reddy, D.S. Mass Spectrometric Assay and Physiological-Pharmacological Activity of Androgenic Neurosteroids. Neurochem. Int. 2008, 52, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.S. Role of Hormones and Neurosteroids in Epileptogenesis. Front. Cell. Neurosci. 2013, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, D.S. Neurosteroids. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 186, pp. 113–137. ISBN 9780444536303. [Google Scholar]
- Baulieu, E.E.; Schumacher, M. Progesterone as a Neuroactive Neurosteroid, with Special Reference to the Effect of Progesterone on Myelination. Hum. Reprod. 2000, 15 (Suppl. 1), 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen Receptors in the Central Nervous System and Their Implication for Dopamine-Dependent Cognition in Females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.P.; Han, W. Regulation of Synaptic Functions in Central Nervous System by Endocrine Hormones and the Maintenance of Energy Homoeostasis. Biosci. Rep. 2012, 32, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Karpinski, M.; Mattina, G.F.; Steiner, M. Effect of Gonadal Hormones on Neurotransmitters Implicated in the Pathophysiology of Obsessive-Compulsive Disorder: A Critical Review. Neuroendocrinology 2017, 105, 1–16. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex Hormones Affect Neurotransmitters and Shape the Adult Female Brain during Hormonal Transition Periods. Front. Neurosci. 2015, 9, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, L.M.; Cornil, C.A.; Mittelman-Smith, M.A.; Rainville, J.R.; Remage-Healey, L.; Sinchak, K.; Micevych, P.E. Actions of Steroids: New Neurotransmitters. J. Neurosci. 2016, 36, 11449–11458. [Google Scholar] [CrossRef] [Green Version]
- Belanoff, J.K.; Gross, K.; Yager, A.; Schatzberg, A.F. Corticosteroids and Cognition. J. Psychiatr. Res. 2001, 35, 127–145. [Google Scholar] [CrossRef]
- Wolf, O.T. Cognitive Functions and Sex Steroids. Ann. Endocrinol. 2003, 64, 158–161. [Google Scholar]
- Ali, S.A.; Begum, T.; Reza, F. Hormonal Influences on Cognitive Function. Malaysian J. Med. Sci. 2018, 25, 31–41. [Google Scholar] [CrossRef]
- Frick, K.M.; Kim, J. Mechanisms Underlying the Rapid Effects of Estradiol and Progesterone on Hippocampal Memory Consolidation in Female Rodents. Horm. Behav. 2018, 104, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. Steroid Hormones: Effect on Brain Development and Function. Horm. Res. 1992, 37, 1–10. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Schmidt, P.J. Gonadal Steroids, Brain, and Behavior: Role of Context. Dialogues Clin. Neurosci. 2002, 4, 123–137. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Membrane Progesterone Receptors: Evidence for Neuroprotective, Neurosteroid Signaling and Neuroendocrine Functions in Neuronal Cells. Neuroendocrinology 2012, 96, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Pang, Y. Anti-Apoptotic Actions of Allopregnanolone and Ganaxolone Mediated Through Membrane Progesterone Receptors (PAQRs) in Neuronal Cells. Front. Endocrinol. 2020, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.J.; Geoghegan, T.E.; Prough, R.A.; Michael Miller, K.K. The Biological Actions of Dehydroepiandrosterone Involves Multiple Receptors. Drug Metab. Rev. 2006, 38, 89–116. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P. Neuroactive Steroid Regulation of Neurotransmitter Release in the CNS: Action, Mechanism and Possible Significance. Prog. Neurobiol. 2009, 89, 134–152. [Google Scholar] [CrossRef]
- Yadid, G.; Sudai, E.; Maayan, R.; Gispan, I.; Weizman, A. The Role of Dehydroepiandrosterone (DHEA) in Drug-Seeking Behavior. Neurosci. Biobehav. Rev. 2010, 35, 303–314. [Google Scholar] [CrossRef]
- Lösel, R.; Wehling, M. Nongenomic Actions of Steroid Hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef]
- Tuem, K.B.; Atey, T.M. Neuroactive Steroids: Receptor Interactions and Responses. Front. Neurol. 2017, 8, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Liu, Y.; Cao, J.-M. G Protein-Coupled Receptors: Extranuclear Mediators for the Non-Genomic Actions of Steroids. Int. J. Mol. Sci. 2014, 15, 15412–15425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The Structure and Function of G-Protein-Coupled Receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR Drug Discovery: New Agents, Targets and Indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Dascal, N.; Kahanovitch, U. The Roles of Gβγ and Gα in Gating and Regulation of GIRK Channels. Int. Rev. Neurobiol. 2015, 123, 27–85. [Google Scholar] [CrossRef] [PubMed]
- Randáková, A.; Nelic, D.; Ungerová, D.; Nwokoye, P.; Su, Q.; Doležal, V.; El-Fakahany, E.E.; Boulos, J.; Jakubík, J. Novel M 2 -selective, G i -biased Agonists of Muscarinic Acetylcholine Receptors. Br. J. Pharmacol. 2020, 177, 2073–2089. [Google Scholar] [CrossRef]
- Randáková, A.; Jakubík, J. Functionally Selective and Biased Agonists of Muscarinic Receptors. Pharmacol. Res. 2021, 169, 105641. [Google Scholar] [CrossRef]
- Li, H.; Papadopoulos, V. Peripheral-Type Benzodiazepine Receptor Function in Cholesterol Transport. Identification of a Putative Cholesterol Recognition/Interaction Amino Acid Sequence and Consensus Pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef]
- Jafurulla, M.; Tiwari, S.; Chattopadhyay, A. Identification of Cholesterol Recognition Amino Acid Consensus (CRAC) Motif in G-Protein Coupled Receptors. Biochem. Biophys. Res. Commun. 2011, 404, 569–573. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Carter, P.A.; Peters, S.C.; Zhang, W.; Ward, J.S.; Mitch, C.H.; Calligaro, D.O.; Whitesitt, C.A.; DeLapp, N.; Shannon, H.E.; et al. Xanomeline Compared to Other Muscarinic Agents on Stimulation of Phosphoinositide Hydrolysis in Vivo and Other Cholinomimetic Effects. Brain Res 1998, 795, 179–190. [Google Scholar] [CrossRef]
- DeLapp, N.; Wu, S.; Belagaje, R.; Johnstone, E.; Little, S.; Shannon, H.; Bymaster, F.; Calligaro, D.; Mitch, C.; Whitesitt, C.; et al. Effects of the M1 Agonist Xanomeline on Processing of Human Beta-Amyloid Precursor Protein (FAD, Swedish Mutant) Transfected into Chinese Hamster Ovary-M1 Cells. Biochem. Biophys. Res. Commun. 1998, 244, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, A.; Pierce, T.L.; Sorman, J.L.; El-Fakahany, E.E. On the Unique Binding and Activating Properties of Xanomeline at the M1 Muscarinic Acetylcholine Receptor. Mol. Pharmacol. 1998, 53, 1120–1130. [Google Scholar] [PubMed]
- Grant, M.K.O.; El-Fakahany, E.E. Persistent Binding and Functional Antagonism by Xanomeline at the Muscarinic M5 Receptor. J. Pharmacol. Exp. Ther. 2005, 315, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avissar, S.; Egozi, Y.; Sokolovsky, M. Studies on Muscarinic Receptors in Mouse and Rat Hypothalamus: A Comparison of Sex and Cyclical Differences. Neuroendocrinology 1981, 32, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Giles, A.; Wilkinson, D.A. M 2 Muscarinic ([3 H] N -Methyl Scopolamine) Binding in Micropunches of Rat Ventricular Myocardium: Characterization and Modification by Progesterone. Can. J. Physiol. Pharmacol. 1992, 70, 943–948. [Google Scholar] [CrossRef]
- Wilkinson, M.; Siauw, M.; Horackova, M. Modulation of cardiac M2 muscarinic receptor binding by progesterone-related steroids. J. Mol. Cell. Cardiol. 1995, 27, 1831–1839. [Google Scholar] [CrossRef]
- Shiraishi, M.; Minami, K.; Shibuya, I.; Uezono, Y.; Ogata, J.; Okamoto, T.; Murasaki, O.; Kaibara, M.; Ueta, Y.; Shigematsu, A. The Inhibitory Effects of Alphaxalone on M1 and M3 Muscarinic Receptors Expressed in Xenopus Oocytes. Anesth. Analg. 2003, 97, 449–455. [Google Scholar] [CrossRef]
- Sokolovsky, M.; Egozi, Y.; Avissar, S. Molecular Regulation of Receptors: Interaction of Beta-Estradiol and Progesterone with the Muscarinic System. Proc. Natl. Acad. Sci. USA 1981, 78, 5554–5558. [Google Scholar] [CrossRef] [Green Version]
- Al-Daham, M.I.M.; Thomas, P.J. Contrasting Effects of Testicular and Ovarian Steroids upon Muscarinic Binding Sites in the Brain. Pharmacology 1987, 34, 250–258. [Google Scholar] [CrossRef]
- Bae, Y.J.; Zeidler, R.; Baber, R.; Vogel, M.; Wirkner, K.; Loeffler, M.; Ceglarek, U.; Kiess, W.; Körner, A.; Thiery, J.; et al. Reference Intervals of Nine Steroid Hormones over the Life-Span Analyzed by LC-MS/MS: Effect of Age, Gender, Puberty, and Oral Contraceptives. J. Steroid Biochem. Mol. Biol. 2019, 193, 105409. [Google Scholar] [CrossRef]
- Hill, M.; Hána, V.; Velíková, M.; Pařízek, A.; Kolátorová, L.; Vítků, J.; Škodová, T.; Šimková, M.; Šimják, P.; Kancheva, R.; et al. A Method for Determination of One Hundred Endogenous Steroids in Human Serum by Gas Chromatography-Tandem Mass Spectrometry. Physiol. Res. 2019, 68, 179–207. [Google Scholar] [CrossRef] [PubMed]
- Lazareno, S.; Popham, A.; Birdsall, N.J. Allosteric Interactions of Staurosporine and Other Indolocarbazoles with N-[Methyl-(3)H]Scopolamine and Acetylcholine at Muscarinic Receptor Subtypes: Identification of a Second Allosteric Site. Mol. Pharmacol. 2000, 58, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazareno, S.; Popham, A.; Birdsall, N.J.M. Analogs of WIN 62,577 Define a Second Allosteric Site on Muscarinic Receptors. Mol. Pharmacol. 2002, 62, 1492–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, A.C.; Ring, A.M.; Manglik, A.; Hu, J.; Hu, K.; Eitel, K.; Hübner, H.; Pardon, E.; Valant, C.; Sexton, P.M.; et al. Activation and Allosteric Modulation of a Muscarinic Acetylcholine Receptor. Nature 2013, 504, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-P.; Prilla, S.; Mohr, K.; Ellis, J. Critical Amino Acid Residues of the Common Allosteric Site on the M2 Muscarinic Acetylcholine Receptor: More Similarities than Differences between the Structurally Divergent Agents Gallamine and Bis(Ammonio)Alkane-Type Hexamethylene-Bis-[Dimethyl-(3-Phthalimidopropyl)ammonium]dibromide. Mol. Pharmacol. 2005, 68, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Leppik, R.A.; Miller, R.C.; Eck, M.; Paquet, J.L. Role of Acidic Amino Acids in the Allosteric Modulation by Gallamine of Antagonist Binding at the M2 Muscarinic Acetylcholine Receptor. Mol. Pharmacol. 1994, 45, 983–990. [Google Scholar]
- Jakubík, J.; El-Fakahany, E.E. Allosteric Modulation of GPCRs of Class A by Cholesterol. Int. J. Mol. Sci. 2021, 22, 1953. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurowska, E.; Szánti-Pintér, E.; Randáková, A.; Jakubík, J.; Kudova, E. Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids. Int. J. Mol. Sci. 2022, 23, 13075. https://doi.org/10.3390/ijms232113075
Szczurowska E, Szánti-Pintér E, Randáková A, Jakubík J, Kudova E. Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids. International Journal of Molecular Sciences. 2022; 23(21):13075. https://doi.org/10.3390/ijms232113075
Chicago/Turabian StyleSzczurowska, Ewa, Eszter Szánti-Pintér, Alena Randáková, Jan Jakubík, and Eva Kudova. 2022. "Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids" International Journal of Molecular Sciences 23, no. 21: 13075. https://doi.org/10.3390/ijms232113075