Ionic Environment Affects Biomolecular Interactions of Amyloid-β: SPR Biosensor Study
Abstract
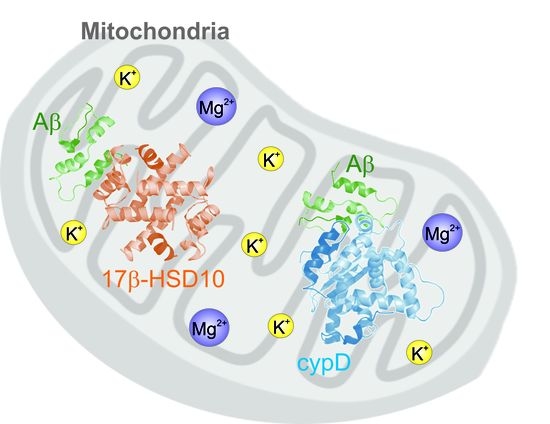
:1. Introduction
2. Results
2.1. Interactions of Aβ1–40 and Aβ1–42 with cypD at Different Concentrations of K+ and Mg2+
2.2. The Effect of the Oligomerization State of Aβ
2.3. Interactions of Aβ1–40 and Aβ1–42 with 17β-HSD10 at Different Concentrations of K+ and Mg2+
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Reagents
4.1.2. Buffers
4.1.3. Preparation of Aβ Samples
4.2. Instrumentation
4.2.1. Surface Plasmon Resonance (SPR) Biosensor
4.2.2. Functionalization of the SPR Chip
4.2.3. Immobilization of cypD to the Functionalized SPR Chip
4.2.4. Immobilization of 17β-HSD10 to the Functionalized SPR Chip
4.3. Interactions of Aβ1–40 and Aβ1–42 with cypD and 17β-HSD10 at Different Concentrations of K+ and Mg2+
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. Jad 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Wang, C. Aβ42 is More Rigid than Aβ40 at the C Terminus: Implications for Aβ Aggregation and Toxicity. J. Mol. Biol. 2006, 364, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Lührs, T. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garai, K.; Frieden, C. Quantitative analysis of the time course of Aβ oligomerization and subsequent growth steps using tetramethylrhodamine-labeled Aβ. Proc. Natl. Acad. Sci. USA 2013, 110, 3321–3326. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009, 218, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Crouch, P.J.; Harding, S.-M.E.; White, A.R.; Camakaris, J.; Bush, A.I.; Masters, C.L. Mechanisms of Aβ mediated neurodegeneration in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2008, 40, 181–198. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer‘s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [Green Version]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. Jad 2018, 64, S567–S610. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Calcium hypothesis of Alzheimer’s disease. Pflügers Arch. -Eur. J. Physiol. 2010, 459, 441–449. [Google Scholar] [CrossRef]
- Sciacca, M.F.; Lolicato, F.; Tempra, C.; Scollo, F.; Sahoo, B.R.; Watson, M.D.; García-Viñuales, S.; Milardi, D.; Raudino, A.; Lee, J.C. Lipid-Chaperone Hypothesis: A Common Molecular Mechanism of Membrane Disruption by Intrinsically Disordered Proteins. Acs Chem. Neurosci. 2020, 11, 4336–4350. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Fang, F.; Chen, D.; Sosunov, A.A.; McKhann, G.M.; Yan, Y.; Wang, C.; Zhang, H.; Molkentin, J.D.; et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008, 14, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Guo, L.; Zhang, W.; Rydzewska, M.; Yan, S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging 2011, 32, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Liu, Y.; Sorci, M.; Belfort, G.; Lustbader, J.W.; Yan, S.S.; Wang, C. Surface Plasmon Resonance and Nuclear Magnetic Resonance Studies of ABAD−Aβ Interaction. Biochemistry 2007, 46, 1724–1731. [Google Scholar] [CrossRef]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.D.; Stern, D.M. Mitochondrial dysfunction and Alzheimer‘s disease: Role of amyloid-β peptide alcohol dehydrogenase (ABAD). Int. J. Exp. Pathol. 2005, 86, 161–171. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, J.; Wang, Y.; Chen, J.; Li, Y.; Duan, Y. An aptamer based method for small molecules detection through monitoring salt-induced AuNPs aggregation and surface plasmon resonance (SPR) detection. Sens. Actuators B Chem. 2016, 236, 474–479. [Google Scholar] [CrossRef]
- Singh, P.; Suman, S.; Chandna, S.; Das, T.K. Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer‘s disease. Bioinformation 2009, 3, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Rao, V.K.; Carlson, E.A.; Yan, S.S. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2014, 1842, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Bartolini, M.; Naldi, M.; Fiori, J.; Valle, F.; Biscarini, F.; Nicolau, D.V.; Andrisano, V. Kinetic characterization of amyloid-beta 1–42 aggregation with a multimethodological approach. Anal. Biochem. 2011, 414, 215–225. [Google Scholar] [CrossRef]
- Hou, L.; Shao, H.; Zhang, Y.; Li, H.; Menon, N.K.; Neuhaus, E.B.; Brewer, J.M.; Byeon, I.-J.L.; Ray, D.G.; Vitek, M.P.; et al. Solution NMR Studies of the Aβ(1−40) and Aβ(1−42) Peptides Establish that the Met35 Oxidation State Affects the Mechanism of Amyloid Formation. J. Am. Chem. Soc. 2004, 126, 1992–2005. [Google Scholar] [CrossRef]
- Wang, Q.; Walsh, D.M.; Rowan, M.J.; Selkoe, D.J.; Anwyl, R. Block of Long-Term Potentiation by Naturally Secreted and Synthetic Amyloid β-Peptide in Hippocampal Slices Is Mediated via Activation of the Kinases c-Jun N-Terminal Kinase, Cyclin-Dependent Kinase 5, and p38 Mitogen-Activated Protein Kinase as well as Metabotropic Glutamate Receptor Type 5. J. Neurosci. 2004, 24, 3370–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittelberger, K.A.; Piazza, F.; Tesco, G.; Reijmers, L.G. Natural Amyloid-Beta Oligomers Acutely Impair the Formation of a Contextual Fear Memory in Mice. PLoS ONE 2012, 7, e29940. [Google Scholar] [CrossRef] [PubMed]
- Hemmerová, E.; Špringer, T.; Krištofiková, Z.; Homola, J. Study of Biomolecular Interactions of Mitochondrial Proteins Related to Alzheimer’s Disease: Toward Multi-Interaction Biomolecular Processes. Biomolecules 2020, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Hemmerová, E.; Špringer, T.; Krištofiková, Z.; Homola, J. In vitro study of interaction of 17β-hydroxysteroid dehydrogenase type 10 and cyclophilin D and its potential implications for Alzheimer’s disease. Sci. Rep. 2019, 9, 16700. [Google Scholar] [CrossRef] [Green Version]
- Krištofiková, Z.; Špringer, T.; Gedeonová, E.; Hofmannová, A.; Říčný, J.; Hromádková, L.; Vyhnálek, M.; Laczo, J.; Nikolai, T.; Hort, J.; et al. Interactions of 17β-Hydroxysteroid Dehydrogenase Type 10 and Cyclophilin D in Alzheimer’s Disease. Neurochem. Res. 2020, 45, 915–927. [Google Scholar]
- Bradshaw, P.C.; Pfeiffer, D.R. Release of Ca2+ and Mg2+ from yeast mitochondria is stimulated by increased ionic strength. Bmc Biochem. 2006, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Haumann, J.; Dash, R.K.; Stowe, D.F.; Boelens, A.D.; Beard, D.A.; Camara, A.K. Mitochondrial free [Ca2+] increases during ATP/ADP antiport and ADP phosphorylation: Exploration of mechanisms. Biophys. J. 2010, 99, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.W.; Apel, L.; Brierley, G.P. Matrix free magnesium changes with metabolic state in isolated heart mitochondria. Biochemistry 1990, 29, 4121–4128. [Google Scholar] [CrossRef]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6, 30027. [Google Scholar] [CrossRef]
- O’Rourke, B.; Cortassa, S.; Aon, M.A. Mitochondrial Ion Channels: Gatekeepers of Life and Death. Physiology 2005, 20, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Garlid, K.D.; Paucek, P. Mitochondrial potassium transport: The K+ cycle. Biochim. Et Biophys. Acta (Bba)-Bioenerg. 2003, 1606, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Kaasik, A.; Safiulina, D.; Zharkovsky, A.; Veksler, V. Regulation of mitochondrial matrix volume. Am. J. Physiol. -Cell Physiol. 2007, 292, C157–C163. [Google Scholar] [CrossRef] [PubMed]
- Augustynek, B.; Wrzosek, A.; Koprowski, P.; Kielbasa, A.; Bednarczyk, P.; Lukasiak, A.; Dolowy, K.; Szewczyk, A. What we don’t know about mitochondrial potassium channels? Postepy Biochem. 2016, 62, 189–198. [Google Scholar] [PubMed]
- Szabò, I.; Leanza, L.; Gulbins, E.; Zoratti, M. Physiology of potassium channels in the inner membrane of mitochondria. Pflügers Arch. -Eur. J. Physiol. 2012, 463, 231–246. [Google Scholar] [CrossRef]
- Zoeteweij, J.P.; van de Water, B.; de Bont, H.J.; Nagelkerke, J.F. Mitochondrial K+ as modulator of Ca(2+)-dependent cytotoxicity in hepatocytes. Novel application of the K(+)-sensitive dye PBFI (K(+)-binding benzofuran isophthalate) to assess free mitochondrial K+ concentrations. Biochem. J. 1994, 299, 539–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [Green Version]
- Gout, E.; Rébeillé, F.; Douce, R.; Bligny, R. Interplay of Mg(2+), ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg(2+) in cell respiration. Proc. Natl. Acad. Sci. USA 2014, 111, E4560–E4567. [Google Scholar] [CrossRef] [Green Version]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxidative Med. Cell. Longev. 2017, 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Dołowy, K.; Szewczyk, A. Matrix Mg2+ regulates mitochondrial ATP-dependent potassium channel from heart. Febs Lett. 2005, 579, 1625–1632. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Yan, S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: Cyclophilin D and amyloid beta. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2010, 1802, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Špringer, T.; Piliarik, M.; Homola, J. Surface plasmon resonance sensor with dispersionless microfluidics for direct detection of nucleic acids at the low femtomole level. Sens. Actuators B Chem. 2010, 145, 588–591. [Google Scholar]
- Špringer, T.; ChadtováSong, X.; Ermini, M.L.; Lamačová, J.; Homola, J. Functional gold nanoparticles for optical affinity biosensing. Anal. Bioanal. Chem. 2017, 409, 4087–4097. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmerová, E.; Špringer, T.; Krištofiková, Z.; Homola, J. Ionic Environment Affects Biomolecular Interactions of Amyloid-β: SPR Biosensor Study. Int. J. Mol. Sci. 2020, 21, 9727. https://doi.org/10.3390/ijms21249727
Hemmerová E, Špringer T, Krištofiková Z, Homola J. Ionic Environment Affects Biomolecular Interactions of Amyloid-β: SPR Biosensor Study. International Journal of Molecular Sciences. 2020; 21(24):9727. https://doi.org/10.3390/ijms21249727
Chicago/Turabian StyleHemmerová, Erika, Tomáš Špringer, Zdeňka Krištofiková, and Jiří Homola. 2020. "Ionic Environment Affects Biomolecular Interactions of Amyloid-β: SPR Biosensor Study" International Journal of Molecular Sciences 21, no. 24: 9727. https://doi.org/10.3390/ijms21249727