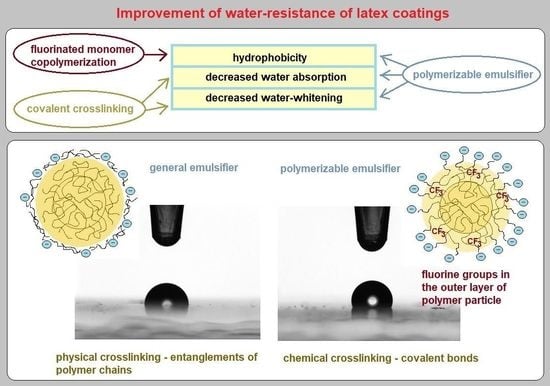
Effect of Fluorinated Comonomer, Polymerizable Emulsifier, and Crosslinking on Water Resistance of Latex Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Testing of Latexes
2.3. Preparation and Testing of Free-Standing Coating Films
2.4. Preparation and Testing of Coatings
3. Results and Discussion
3.1. Characterization of Liquid Latexes and Dried Copolymers
3.2. Water Resistance of Coating Films
3.3. Surface Properties of Latex Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez, R.; Alarcón, C.; Ekanayake, P.; McDonald, P.J.; Keddie, J.L.; Barandiaran, M.J.; Asua, J.M. Correlation of silicone incorporation into hybrid acrylic coatings with the resulting hydrophobic and thermal properties. Macromolecules 2008, 41, 8537–8546. [Google Scholar] [CrossRef] [Green Version]
- Machotová, J.; Černošková, E.; Honzíček, J.; Šňupárek, J. Water sensitivity of fluorine-containing polyacrylate latex coatings: Effects of crosslinking and ambient drying conditions. Prog. Org. Coat. 2018, 120, 266–273. [Google Scholar] [CrossRef]
- Bassett, D.R. Hydrophobic coatings from emulsion polymers. J. Coat. Technol. 2001, 73, 43–55. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, R.; Li, S.; Xiang, T.; Zhao, C.-S. A robust way to prepare blood-compatible and anti-fouling polyethersulfone membrane. Colloids Surf. B Biointerfaces 2016, 146, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Oldani, V.; del Negro, R.; Bianchi, C.L.; Suriano, R.; Turri, S.; Pirola, C.; Sacchi, B. Surface properties and anti-fouling assessment of coatings obtained from perfluoropolyethers and ceramic oxides nanopowders deposited on stainless steel. J. Fluorine Chem. 2015, 180, 7–14. [Google Scholar] [CrossRef]
- Zheng, S.X.; Li, J.H. Inorganic-organic sol gel hybrid coatings for corrosion protection of metals. J. Sol.-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Chiong, S.J.; Goh, P.S.; Ismail, A.F. Novel hydrophobic PVDF/APTES-GO nanocomposite for natural gas pipelines coating. J. Nat. Gas Sci. Eng. 2017, 42, 190–202. [Google Scholar] [CrossRef]
- Zhou, C.L.; Lu, X.; Xin, Z.; Liu, J.; Zhang, Y.F. Hydrophobic benzoxazine-cured epoxy coatings for corrosion protection. Prog. Org. Coat. 2013, 76, 1178–1183. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Il’darkhanova, F.I.; Mironova, G.A.; Bogoslovsky, K.G.; Men’shikov, V.V.; Bykov, E.D. Development of paint coatings with superhydrophobic properties. Prot. Met. Phys. Chem. Surf. 2012, 48, 796–802. [Google Scholar] [CrossRef]
- López, A.B.; de la Cal, J.C.; Asua, J.M. Highly hydrophobic coatings from waterborne latexes. Langmuir 2016, 32, 7459–7466. [Google Scholar] [CrossRef]
- Chen, L.; Shao, T.; Gong, Y.; Wang, X.; Sun, Z. Synthesis and properties of cross-linked fluorine and silicon VAc-VeoVa polymer latex emulsified by green mixed surfactant. J. Polym. Mater. 2018, 35, 281–293. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.; Hao, L.; Zhang, D.; Zhang, M. Synthesis and characterization of self-crosslinking fluorinated polyacrylate soap-free latices with core-shell structure. App. Surf. Sci. 2013, 268, 373–380. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Z.; Chen, L. Preparation of cross-linked and fluorine-silicon modified acrylate emulsion by using mixed green surfactants. J. Polym. Mater. 2016, 33, 685–696. [Google Scholar]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. Effect of surface fluorination of P25-TiO2 coated on nickel substrate for photocatalytic oxidation of methyl ethyl ketone in indoor environments. J. Environ. Chem. Eng. 2019, 7, 103390. [Google Scholar] [CrossRef]
- Zhang, N.; Li, D.; Mu, M.; Lu, M. Partially fluorinated UiO-66-NH2 (Zr): Positive effect of the fluorine moiety on the adsorption capacity for environmental pollutants of metal-organic frameworks. Chem. Eng. J. 2022, 448, 137467. [Google Scholar] [CrossRef]
- Cui, X.; Zhong, S.; Gao, Y.; Wang, H. Preparation and characterization of emulsifier-free core-shell interpenetrating polymer network-fluorinated polyacrylate latex particles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 324, 14–21. [Google Scholar] [CrossRef]
- Cui, X.; Zhong, S.; Wang, H. Emulsifier-free core-shell polyacrylate latex nanoparticles containing fluorine and silicon in shell. Polymer 2007, 48, 7241–7248. [Google Scholar] [CrossRef]
- Anton, D. Surface-fluorinated coatings. Adv. Mater. 1998, 10, 1197–1205. [Google Scholar] [CrossRef]
- Thomas, R.R.; Anton, D.R.; Graham, W.F.; Darmon, M.J.; Stika, K.M. Films containing reactive mixtures of perfluoroalkylethyl methacrylate copolymers and fluorinated isocyanates: Synthesis and surface properties. Macromolecules 1998, 31, 4595–4604. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y. Investigation of fluorinated polyacrylate latex with core-shell structure. Polym. Int. 2005, 54, 1027–1033. [Google Scholar] [CrossRef]
- Morita, M.; Ogisu, H.; Kubo, M. Surface properties of perfluoroalkylethyl acrylate/n-alkyl acrylate copolymers. J. Appl. Polym. Sci. 1999, 73, 1741–1749. [Google Scholar] [CrossRef]
- Schmidt, D.L.; Brady, R.F.; Lam, K.J.; Schmidt, D.C.; Chaudhury, M.K. Contact angle hysteresis, adhesion, and marine biofouling. Langmuir 2004, 20, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Lü, T.; Qi, D.; Zhang, D.; Liu, Q.; Zhao, H. Fabrication of self-cross-linking fluorinated polyacrylate latex particles with core-shell structure and film properties. React. Funct. Polym. 2016, 104, 9–14. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Wang, Y.; Cheng, S.; Chen, P. Study of self-crosslinking acrylate latex containing fluorine. J. Appl. Polym. Sci. 2003, 90, 3609–3616. [Google Scholar] [CrossRef]
- Chen, L.; Bao, Z.; Fu, Z.; Li, W. Preparation and characterisation of novel cross-linked poly (IBOMA-BA-DFMA) latex. Pigment. Resin Technol. 2015, 44, 333–338. [Google Scholar] [CrossRef]
- Chen, L.; Wu, F. Preparation and characterization of novel self cross-linking fluorinated acrylic latex. J. Appl. Polym. Sci. 2012, 123, 1997–2002. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Xu, R. Preparation and surface properties of core-shell polyacrylate latex containing fluorine and silicon in the shell. J. Appl. Polym. Sci. 2011, 119, 1576–1585. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.H.; Zhou, J.W.; Yuan, X.Y. Synthesis and characterization of core-shell polyacrylate latex containing fluorine/silicone in the shell and the selfstratification film. Colloid Polym. Sci. 2012, 290, 203–211. [Google Scholar] [CrossRef]
- Ruckerova, A.; Machotova, J.; Svoboda, R.; Pukova, K.; Bohacik, P.; Valka, R. Ambient temperature self-crosslinking latices using low generation PAMAM dendrimers as inter-particle crosslinking agents. Prog. Org. Coat. 2019, 119, 91–98. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room temperature. Prog. Polym. Sci. 2011, 36, 191–217. [Google Scholar] [CrossRef]
- Gonzáles, I.; Asua, J.M.; Leiza, J.R. Crosslinking in acetoacetoxy functional waterborne crosslinkable latexes. Macromol. Symp. 2006, 243, 53–62. [Google Scholar] [CrossRef]
- Nakayama, Y. Development of novel aqueous coatings which meet the requirements of ecology-conscious society: Novel cross-linking system based on the carbonyl-hydrazide reaction and its applications. Prog. Org. Coat. 2004, 51, 280–299. [Google Scholar] [CrossRef]
- Koukiotis, C.G.; Karabela, M.M.; Sideridou, I.D. Mechanical properties of films of latexes based on copolymers BA/MMA/DAAM and BA/MMA/VEOVA-10/DAAM and the corresponding self-crosslinked copolymers using the adipic acid dihydrazide as crosslinking agent. Prog. Org. Coat. 2012, 75, 106–115. [Google Scholar] [CrossRef]
- Koukiotis, C.; Sideridou, I.D. Synthesis and characterization of latexes based on copolymers BA/MMA/DAAM and BA/MMA/VEOVA-10/DAAM and the corresponding 1K crosslinkable binder using the adipic acid dihydrazide as crosslinking agent. Prog. Org. Coat. 2010, 69, 504–509. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Huang, H.; Li, Y.; Chen, H. The diacetone acrylamide crosslinking reaction and its control of core-shell polyacrylate lattices at ambient temperature. J. Appl. Polym. Sci. 2012, 123, 1822–1832. [Google Scholar] [CrossRef]
- Li, H.; Kan, C.; Du, Y.; Liu, D. Effects of the amount of diacetone acrylamide on the properties of styrene-acrylic copolymer latexes and their films. Polym. Prep. 2002, 43, 413–414. [Google Scholar]
- Kessel, N.; Illsley, D.R.; Keddie, J.L. The diacetone acrylamide crosslinking reaction and its influence on the film formation of an acrylic latex. J. Coat. Technol. Res. 2008, 5, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Kan, C.Y.; Du, Y.; Liu, D.S. Synthesis and properties of soap-free poly(methyl methacrylate-ethyl acrylate-methacrylic acid) latex particles prepared by seeded emulsion polymerization. Eur. Polym. J. 2005, 41, 439–445. [Google Scholar] [CrossRef]
- Xu, G.; Deng, L.; Wen, X.; Pi, P.; Zheng, D.; Cheng, J.; Yang, Z. Synthesis and characterization of fluorine-containing poly-styrene-acrylate latex with core-shell structure using a reactive surfactant. J. Coat. Technol. Res. 2011, 8, 401–407. [Google Scholar] [CrossRef]
- Aramendia, E.; Barandiaran, M.J.; Grade, J.; Blease, T.; Asua, J.M. Polymerization of high-solids-content acrylic latexes using a nonionic polymerizable surfactant. J. Polym. Sci. A Polym. Chem. 2002, 40, 1552–1559. [Google Scholar] [CrossRef]
- Yang, S.F.; Xiong, P.T.; Gong, T.; Lu, D.P.; Guan, R. St-BA copolymer emulsion prepared by using novel cationic maleic dialkyl polymerizable emulsifier. Eur. Polym. J. 2005, 41, 2973–2979. [Google Scholar] [CrossRef]
- Filet, A.; Guillot, J.; Hamaide, T.; Guyot, A. Emulsion copolymerization of styrene with a nonionic styrenic polymerizable surfactant. Polym. Advan. Technol. 1995, 6, 465–472. [Google Scholar] [CrossRef]
- Chern, C.S.; Chen, Y.C. Semibatch emulsion polymerization of butyl acrylate stabilized by a polymerizable surfactant. Polym. J. 1996, 28, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Schoonbrood, H.A.S.; Asua, J.M. Reactive surfactants in heterophase polymerization. 9. Optimum surfmer behaviour in emulsion polymerization. Macromolecules 1997, 30, 6034–6041. [Google Scholar] [CrossRef]
- Guyot, A. Advances in reactive surfactants. Adv. Colloid Interface Sci. 2004, 108–109, 3–22. [Google Scholar] [CrossRef]
- Hellgren, A.C.; Weissenborn, P.; Holmberg, K. Surfactants in water-borne paints. Prog. Org. Coat. 1999, 35, 79–87. [Google Scholar] [CrossRef]
- Chern, C.S.; Chen, Y.C. Stability of the polymerizable surfactant stabilized latex particles during semibatch emulsion polymerization. Colloid Polym. Sci. 1997, 275, 124–130. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y. Emulsion polymerization of fluorinated acrylate in the presence of a polymerizable emulsifier. Colloids Surf. A: Physicochem. Eng. Asp. 2009, 348, 151–156. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, C.Z.; Song, Y.; Zhang, Y.Q. Preparation and characterization of novel acrylate emulsiones with ketone-hydrazide crosslinking structure based on application of reactive emulsifier. Adv. Mat. Res. 2013, 833, 335–338. [Google Scholar] [CrossRef]
- Hao, L.; An, Q.; Xu, W.; Zhang, D.; Zhang, M. Effect of polymerizable emulsifier and fluorine monomer on properties of self-crosslinking fluorinated polyacrylate soap-free latexes. J. Polym. Res. 2013, 20, 174. [Google Scholar] [CrossRef]
- Ugelstad, J.; El-Aasser, M.S.; Vanderhoff, J.W. Emulsion polymerization: Initiation of polymerization in monomer droplets. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 503–513. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, Z.; Shi, T.; Wang, H. Synthesis and characterization of core-shell LIPN-fluorine-containing polyacrylate latex. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 119–124. [Google Scholar] [CrossRef]
- Ha, J.K.; Park, I.J. Preparation and characterization of core-shell particles containing perfluoroalkyl acrylate in the shell. Macromolecules 2002, 35, 6811–6818. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.F.; Hao, L.F.; Huang, L.X. Synthesis, film morphology and performance of cationic fluorinated polyacrylate emulsion with core-shell structure. J. Appl. Polym. Sci. 2012, 125, 2376–2383. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, Y.; Chen, Z. Core-shell latex containing fluorine polymer rich in shell. J. Appl. Polym. Sci. 2002, 85, 1147–1153. [Google Scholar] [CrossRef]
- Jong, W.; In, J.P.; Kim, D.K. Surface properties of cote-shell particles containing perfluoroalkyl acrylate in shell. Surf. Sci. 2003, 328, 532–535. [Google Scholar]
- Gao, J.; Wang, X. Synthesis and characterization of novel fluorine-containing polymer emulsion with core/shell structure. J. Fluor. Chem. 2006, 127, 282–286. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. 2nd-Order transition temperatures and related properties of polystyrene. 1. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Al Islam, M.A.; Rahman, A.F.M.M.; Iftekhar, S.; Salem, K.S.; Sultana, N.; Bari, M.L. Morphology, thermal stability, electrical, and mechanical properties of graphene incorporated poly (vinyl alcohol)-gelatin nanocomposites. Int. J. Compos. Mater. 2016, 6, 172–182. [Google Scholar]
- Salem, K.S.; Lubna, M.M.; Rahman, A.M.; NurNabi, M.; Islam, R.; Khan, M.A. The effect of multiwall carbon nanotube additions on the thermo-mechanical, electrical, and morphological properties of gelatin-polyvinyl alcohol blend nanocomposite. J. Compos. Mater. 2015, 49, 1379–1391. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 427–473. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Tobing, S.; Klein, A. Molecular parameters and their relation to the adhesive performance of acrylic pressure-sensitive adhesives. J. Appl. Polym. Sci. 2001, 79, 2230–2244. [Google Scholar] [CrossRef]
- Vandenburg, H.J.; Clifford, A.A.; Bartle, K.D.; Carlson, R.E.; Caroll, J.; Newton, I.D. A simple solvent selection method accelerated solvent extraction of additives from polymers. Analyst 1999, 124, 1707–1710. [Google Scholar] [CrossRef]
- Knotek, P.; Chanova, E.; Rypacek, F. AFM Imaging and analysis of local mechanical properties for detection of surface pattern of functional groups. Mat. Sci. Eng. C 2013, 33, 1963–1968. [Google Scholar] [CrossRef]
- Todorov, R.; Lozanova, V.; Knotek, P.; Černošková, E.; Vlček, M. Microstructure and ellipsometric modelling of the optical properties of very thin silver films for application in plasmonics. Thin Solid Films 2017, 628, 22–30. [Google Scholar] [CrossRef]
- Knotek, P.; Tichý, L. Atomic force microscopy and atomic force acoustic microscopy characterization of photo-induced changes in some Ge-As-S amorphous films. Thin Solid Films 2009, 517, 1837–1840. [Google Scholar] [CrossRef]
- Knotek, P.; Arsova, D.; Vateva, E.; Tichy, L. Photo-expansion in Ge-As-S amorphous film monitored by digital holographic microscopy and atomic force microscopy. J. Optoelectron. Adv. Mater. 2009, 11, 391–394. [Google Scholar]
- Aguirreurreta, Z.; Cal, J.C.; Leiza, J.R. Anionic polymerizable surfactants and stabilizers in emulsion polymerization: A comparative study. Macromol. React. Eng. 2017, 11, 1600033. [Google Scholar] [CrossRef] [Green Version]
- Mekki, S.; Saïdi-Besbes, S.; Elaïssari, A.; Valour, J.P.; Derdour, A. Novel polymerizable surfactants: Synthesis and application in the emulsion polymerization of styrene. Polym. J. 2010, 42, 401–405. [Google Scholar] [CrossRef]
- Urquiola, M.B.; Dimonie, V.L.; Sudol, E.D.; El-Aasser, M.S. Emulsion polymerization of vinyl acetate using a polymerizable surfactant. I. Kinetic studies. J. Appl. Polym. Sci. A Polym. Chem. 1992, 30, 2619–2629. [Google Scholar] [CrossRef]
- Pi, P.; Wang, W.; Wen, X.; Xu, S.; Cheng, J. Synthesis and characterization of low-temperature self-crosslinkable acrylic emulsion for PE film ink. Prog. Org. Coat. 2015, 81, 66–71. [Google Scholar] [CrossRef]
- Podzimek, S.; Machotova, J.; Snuparek, J.; Vecera, M.; Prokupek, L. Characterization of molecular structure of acrylic copolymers prepared via emulsion polymerization using A4F-MALS technique. J. Appl. Polym. Sci. 2014, 131, 11178–11185. [Google Scholar] [CrossRef]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Alberdi, J.M.; Schoonbrood, H.A.; Charmot, D.; Asua, J.M. Seeded semibatch emulsion polymerization of butyl acrylate: Effect of the chain-transfer agent on the kinetics and structural properties. J. Polym. Sci. A Polym. Chem. 2001, 39, 1106–1119. [Google Scholar] [CrossRef]
- Machotová, J.; Kalendová, A.; Steinerová, D.; Mácová, P.; Šlang, S.; Šňupárek, J.; Vajdák, J. Water-resistant latex coatings: Tuning of properties by polymerizable surfactant, covalent crosslinking and nanostructured ZnO additive. Coatings 2021, 11, 347. [Google Scholar] [CrossRef]
- Winnik, M.A. Interdiffusion and crosslinking in thermoset latex films. J. Coat. Technol. 2002, 74, 49–63. [Google Scholar] [CrossRef]
- Taylor, J.W.; Winnik, M.A. Functional latex and thermoset latex films. JCT Res. 2004, 1, 163–190. [Google Scholar] [CrossRef]
- Tian, Y.; Du, E.; Abdelmola, F.; Qiang, Y.; Carlsson, L. A Rapid characterization of water diffusion in polymer specimens using a droplet-based method. Langmuir 2020, 36, 7309–7314. [Google Scholar] [CrossRef]
- Machotová, J.; Kalendová, A.; Zlámaná, B.; Šňupárek, J.; Palarčík, J.; Svoboda, R. Waterborne coating binders based on self-crosslinking acrylic latex with embedded inorganic nanoparticles: A comparison of nanostructured ZnO and MgO as crosslink density enhancing agents. Coatings 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Tsavalas, J.G.; Sundberg, D.C. Water whitening of polymer films: Mechanistic studies and comparison between water and solvent borne films. Prog. Org. Coat. 2017, 105, 56–66. [Google Scholar] [CrossRef]
- Liu, Y.; Gajawicz, A.M.; Rodin, V.; Soer, W.; Scheerder, J.; Satgurunathan, G.; McDonald, P.J.; Keddie, J.L. Explanations for water whitening in secondary dispersion and emulsion polymer films. J. Polym. Sci. B Polym. Phys. 2016, 54, 1658–1674. [Google Scholar] [CrossRef] [Green Version]
- Tsavalas, J.G.; Sundberg, D.C. Hydroplasticization of polymers: Model predictions and application to emulsion polymers. Langmuir 2010, 26, 6960–6966. [Google Scholar] [CrossRef]
- Cerveny, S.; Swenson, J. Water dynamics in the hydration shells of biological and non-biological polymers. J. Chem. Phys. 2019, 150, 234904. [Google Scholar] [CrossRef]
- Salem, K.S.; Starkey, H.R.; Pal, L.; Lucia, L.; Jameel, H. The topochemistry of cellulose nanofibrils as a function of mechanical generation energy. ACS Sustain. Chem. Eng. 2019, 8, 1471–1478. [Google Scholar] [CrossRef]
- Salem, K.S.; Naithani, V.; Jameel, H.; Lucia, L.; Pal, L. A systematic examination of the dynamics of water-cellulose interactions on capillary force-induced fiber collapse. Carbohydr. Polym. 2022, 295, 119856. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Eleftheriou, M.; Zhou, R. Hydration and dewetting near fluorinated superhydrophobic plates. J. Am. Chem. Soc. 2006, 128, 12439–12447. [Google Scholar] [CrossRef]
- Sopha, H.; Tesar, K.; Knotek, P.; Jäger, A.; Hromadko, L.; Macak, J.M. TiO2 nanotubes grown on Ti substrates with different microstructure. Mater. Res. Bull. 2018, 103, 197–204. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Dutil, I.; Gauquelin, L.; Yan, N.; Farnood, R.R. Preparation of self-healing acrylic latex coatings using novel oil-filled ethyl cellulose microcapsules. Prog. Org. Coat. 2015, 85, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Ataei, S.; Khorasani, S.N.; Neisiany, R.E. Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Prog. Org. Coat. 2019, 129, 77–95. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, C.; Camilo, R.P.; Zhang, H.; Cobaj, A.; Soucek, M.D.; Zacharia, N.S. Self-healing latex containing polyelectrolyte multilayers. Macromol. Mater. Eng. 2018, 303, 1700596. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, M.; de Vos, K.; Kastelijn, M.; Peters, R.A.H.; Loos, J.; Scheerder, J. Recovery dynamics of acrylic coating surfaces under elevated relative humidity monitored by atomic force microscopy. Prog. Org. Coat. 2020, 146, 105712. [Google Scholar] [CrossRef]







| Emulsifier | Type | Chemical Composition | Active Matter (wt.%) | Critical Micelle Concentration (mg/L) a |
|---|---|---|---|---|
| Disponil FES 993 | General | Sodium salt of fatty alcohol polyglycol ether sulfate | 29.7 | 200 |
| HITENOL AR-10 | Polymerizable | Ammonium salt of polyoxyethylene styrenated propenyl phenyl ether sulfate | 99.1 | 250 |
| Sample | Composition of Monomer Feeds (g) | Emulsifier Type | Fluorination of the Second-Step Copolymer | |
|---|---|---|---|---|
| TFEMA/MMA/BA/MAA/DAAM/ALMA | ||||
| First Step | Second Step | |||
| Series 1: No crosslinking | ||||
| D1 | 0/18.5/30/1.5/0/0 | 0/18.5/30/1.5/0/0 | general | no |
| H1 | 0/18.5/30/1.5/0/0 | 0/18.5/30/1.5/0/0 | polymerizable | no |
| D1_F | 0/18.5/30/1.5/0/0 | 15/6.5/27/1.5/0/0 | general | yes |
| H1_F | 0/18.5/30/1.5/0/0 | 15/6.5/27/1.5/0/0 | polymerizable | yes |
| Series 2: Keto-hydrazide crosslinking | ||||
| D2 | 0/18.5/30/1.5/0/0 | 0/16.5/29.5/1.5/2.5/0 | general | no |
| H2 | 0/18.5/30/1.5/0/0 | 0/16.5/29.5/1.5/2.5/0 | polymerizable | no |
| D2_F | 0/18.5/30/1.5/0/0 | 15/4.5/26.5/1.5/2.5/0 | general | yes |
| H2_F | 0/18.5/30/1.5/0/0 | 15/4.5/26.5/1.5/2.5/0 | polymerizable | yes |
| Series 3: ALMA crosslinking | ||||
| D3 | 0/18/30/1.5/0/0.5 | 0/18.5/30/1.5/0/0 | general | no |
| H3 | 0/18/30/1.5/0/0.5 | 0/18.5/30/1.5/0/0 | polymerizable | no |
| D3_F | 0/18/30/1.5/0/0.5 | 15/6.5/27/1.5/0/0 | general | yes |
| H3_F | 0/18/30/1.5/0/0.5 | 15/6.5/27/1.5/0/0 | polymerizable | yes |
| Series 4: Keto-hydrazide and ALMA crosslinking | ||||
| D4 | 0/18/30/1.5/0/0.5 | 0/16.5/29.5/1.5/2.5/0 | general | no |
| H4 | 0/18/30/1.5/0/0.5 | 0/16.5/29.5/1.5/2.5/0 | polymerizable | no |
| D4_F | 0/18/30/1.5/0/0.5 | 15/4.5/26.5/1.5/2.5/0 | general | yes |
| H4_F | 0/18/30/1.5/0/0.5 | 15/4.5/26.5/1.5/2.5/0 | polymerizable | yes |
| Component | Reaction Flask (g) | First-Step Monomer Emulsion (g) | Second-Step Monomer Emulsion (g) |
|---|---|---|---|
| Distilled water | 33.00 | 50.00 | 72.00 |
| Disponil FES 993 a | 0.24 | 3.70 | 3.70 |
| HITENOL AR-10 b | 0.07 | 1.10 | 1.10 |
| Ammonium persulfate | 0.20 | 0.20 | 0.20 |
| Monomers | 0.00 | 50.00 | 50.00 |
| Sample | After the First Step | After the Completed Polymerization | ||
|---|---|---|---|---|
| Particle Diameter (nm) | Polydispersity Index (%) | Particle Diameter (nm) | Polydispersity Index (%) | |
| Series 1: No crosslinking | ||||
| D1 | 96.3 ± 0.6 | 5.3 ± 3.6 | 123.4 ± 0.8 | 3.4 ± 2.8 |
| H1 | 90.6 ± 0.4 | 4.2 ± 3.8 | 114.3 ± 0.9 | 5.3 ± 3.9 |
| D1_F | 95.8 ± 1.0 | 3.8 ± 2.0 | 122.4 ± 0.7 | 2.6 ± 1.7 |
| H1_F | 91.4 ± 0.8 | 4.4 ± 2.8 | 116.8 ± 1.2 | 4.6 ± 3.2 |
| Series 2: Keto-hydrazide crosslinking | ||||
| D2 | 97.1 ± 0.9 | 3.2 ± 2.6 | 120.4 ± 1.0 | 3.4 ± 2.3 |
| H2 | 87.8 ± 1.1 | 2.6 ± 1.4 | 112.3± 1.0 | 4.3 ± 2.8 |
| D2_F | 103.1 ± 1.3 | 5.1 ± 4.0 | 128.6 ± 1.2 | 2.4 ± 1.6 |
| H2_F | 96.3 ± 0.7 | 3.5 ± 1.9 | 120.2 ± 1.4 | 5.0 ± 3.2 |
| Series 3: ALMA crosslinking | ||||
| D3 | 96.8 ± 1.2 | 3.4 ± 3.1 | 123.7 ± 1.0 | 2.8 ± 1.9 |
| H3 | 89.9 ± 1.0 | 4.6 ± 4.0 | 114.7 ± 0.7 | 2.5 ± 1.4 |
| D3_F | 98.2 ± 1.6 | 3.8 ± 2.4 | 124.8 ± 1.1 | 2.6 ± 1.7 |
| H3_F | 92.4 ± 0.9 | 4.2 ± 2.7 | 118.4 ± 1.5 | 5.2 ± 4.0 |
| Series 4: Keto-hydrazide and ALMA crosslinking | ||||
| D4 | 95.1 ± 1.1 | 4.3 ± 3.1 | 118.3 ± 1.2 | 4.8 ± 4.6 |
| H4 | 82.0 ± 1.2 | 5.6 ± 4.4 | 105.7 ± 1.1 | 2.6 ± 1.8 |
| D4_F | 97.5 ± 1.5 | 5.8 ± 4.0 | 121.1 ± 1.1 | 3.3 ± 2.5 |
| H4_F | 80.9 ± 0.6 | 4.8 ± 3.7 | 102.5 ± 0.8 | 4.2 ± 3.2 |
| Sample | Mc (g/mol) | Crosslink Density × 10−6 (moles/cm3) |
|---|---|---|
| Series 1: No crosslinking | ||
| D1 | NA a | NA a |
| H1 | 240,600 ± 27,000 | 4.3 ± 0.6 |
| D1_F | NA a | NA a |
| H1_F | 148,200 ± 11,900 | 7.5 ± 0.6 |
| Series 2: Keto-hydrazide crosslinking | ||
| D2 | 58,500 ± 700 | 18.8 ± 0.2 |
| H2 | 50,700 ± 2500 | 21.8 ± 1.1 |
| D2_F | 44,600 ± 5300 | 24.9 ± 3.0 |
| H2_F | 35,200 ± 3400 | 31.5 ± 3.1 |
| Series 3: ALMA crosslinking | ||
| D3 | 18,100 ± 2500 | 61.7 ± 8.8 |
| H3 | 6800 ± 200 | 162.1 ± 5.1 |
| D3_F | 12,900 ± 900 | 85.7 ± 6.2 |
| H3_F | 6300 ± 300 | 175.0 ± 7.7 |
| Series 4: Keto-hydrazide and ALMA crosslinking | ||
| D4 | 5600 ± 200 | 197.8 ± 4.3 |
| H4 | 3800 ± 100 | 289.3 ± 4.6 |
| D4_F | 3400 ± 200 | 322.5 ± 17.4 |
| H4_F | 2800 ± 50 | 392.2 ± 5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machotova, J.; Knotek, P.; Cernoskova, E.; Svoboda, R.; Zarybnicka, L.; Kohl, M.; Kalendova, A. Effect of Fluorinated Comonomer, Polymerizable Emulsifier, and Crosslinking on Water Resistance of Latex Coatings. Coatings 2022, 12, 1150. https://doi.org/10.3390/coatings12081150
Machotova J, Knotek P, Cernoskova E, Svoboda R, Zarybnicka L, Kohl M, Kalendova A. Effect of Fluorinated Comonomer, Polymerizable Emulsifier, and Crosslinking on Water Resistance of Latex Coatings. Coatings. 2022; 12(8):1150. https://doi.org/10.3390/coatings12081150
Chicago/Turabian StyleMachotova, Jana, Petr Knotek, Eva Cernoskova, Roman Svoboda, Lucie Zarybnicka, Miroslav Kohl, and Andrea Kalendova. 2022. "Effect of Fluorinated Comonomer, Polymerizable Emulsifier, and Crosslinking on Water Resistance of Latex Coatings" Coatings 12, no. 8: 1150. https://doi.org/10.3390/coatings12081150