- 1Department of Physiology and Cellular Biophysics, Columbia University Medical Center, New York, NY, United States
- 2Department of Evolutionary Anthropology, Duke University, Durham, NC, United States
- 3Marrow Adiposity and Bone Lab (MAB Lab) ULR4490, Univ Littoral Côte d’Opale, Boulogne-sur-Mer, France
- 4Laboratory of Regenerative Hematopoiesis, Institute of Bioengineering, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
- 5Department of Biomedical Sciences, University of Lausanne, Lausanne, Switzerland
- 6Molecular Physiology of Bone, Institute of Physiology of the Czech Academy of Sciences, Prague 4, Czechia
- 7Section of Endocrinology, Department of Internal Medicine, Center for Bone Quality, Leiden University Medical Center, Leiden, Netherlands
- 8Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy
The first International Summer School on Bone Marrow Adiposity was organized by members of Bone Marrow Adiposity Society and held virtually on September 6-8 2021. The goal of this meeting was to bring together young scientists interested in learning about bone marrow adipose tissue biology and pathology. Fifty-two researchers from different backgrounds and fields, ranging from bone physiopathology to adipose tissue biology and hematology, participated in the summer school. The meeting featured three keynote lectures on the fundamentals of bone marrow adiposity, three scientific workshops on technical considerations in studying bone marrow adiposity, and six motivational and career development lectures, spanning from scientific writing to academic career progression. Moreover, twenty-one participants presented their work in the form of posters. In this report we highlight key moments and lessons learned from the event.
Background
The growing interest in bone marrow adipose tissue (BMAT) is evident from the increase in articles published yearly by researchers all over the world (Figure 1). The credit for bringing this field to international attention is given to Dr. Mehdi Tavassoli with his works in the early 70’s (1), although observations of the transformation of red marrow into yellow marrow have been published since the 1800’s (2, 3). In the last twenty years, the study of BMAT has emerged as an important field in bone and bone marrow biology.
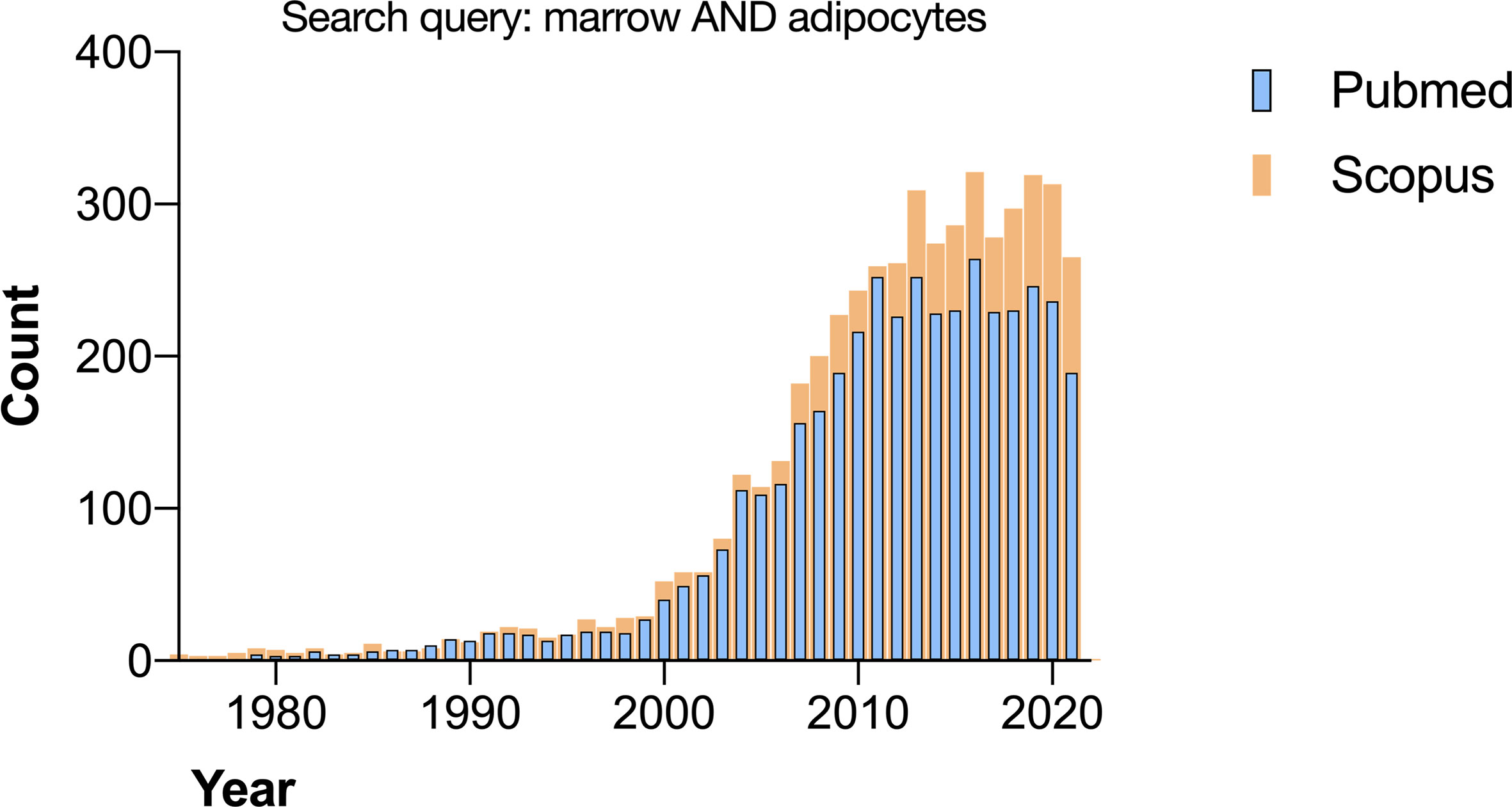
Figure 1 Search results for “marrow AND adipocytes” on Pubmed and Scopus performed in November 2021.
Beginning in 2015, international meetings on Bone Marrow Adiposity (BMA) have been organized with the aim to bring together scientists working on or interested in BMAT (4–8). Later in 2017, a group of BMA scientists founded the BMA Society (BMAS) (http://bma-society.org/) with Dr. Pierre Hardouin as the founding president. The aims of the newly established society were to advance the knowledge of BMA by facilitating interdisciplinary exchanges, developing research strategies, and standardizing nomenclature and methods to study BMAT. Thus far, the society has produced and published three consensus articles (9–11).
One of BMAS’s key goals is to promote BMAT research by training and supporting young scientists interested in this field. Therefore, a group of young scientists from Europe and the United States envisioned the first BMAS Summer School, which was held as a virtual meeting on September 6-8, 2021. The goal of the event was to foster general knowledge of BMAT research to young researchers from all fields in order to provide fundamentals about how BMAT originates, develops, and interacts with other organs, as well as how to study BMA and what methods to use in both clinical and basic research. Moreover, the event program contained career development workshops with the aim to provide tips on how to face difficulties and overcome obstacles in the academic life, which alternative paths exist outside academia, and how to write a good manuscript and how to understand where to publish. Fifty-two participants from all over the world (Figure 2) attended the meeting, with active participation, live discussion, and incredible enthusiasm.

Figure 2 Word cloud performed with the countries of origin of the attendees at the 1st BMAS Summer School. The number of attendees from each country correlates with the word size in the cloud. The higher the number of attendees, the bigger the word. The analysis was carried out with WordCloud package in R.
The three-day event consisted of a daily keynote lecture on BMA, followed by a scientific workshop on how to study BMA and two motivational and career development workshops with a total of twelve presentations from invited speakers. Moreover, twenty-one abstracts were selected and presented as posters with a live discussion; these abstracts have been published and are available elsewhere (12).
Lectures on Bone Marrow Adiposity
BMAT: Origin, Multicellularity, Regulation of Lineage Commitment, and Functional Implications
Dr. Moustapha Kassem (University of Southern Denmark) opened the three-day event with a lecture entitled “BMAT: origin, multicellularity, regulation of lineage commitment, and functional implications”. In his talk, Dr. Kassem described a historical roadmap from the first correlations with bone diseases, such as osteoporosis, to the most recent studies on the regulation of bone marrow stromal cell differentiation potential towards osteoblasts or bone marrow adipocytes. This session was moderated by Dr. Gustavo Duque (University of Melbourne). Dr. Kassem gave an overview of the major findings and discoveries on BMA in relation to bone health and homeostasis and its relevance to clinical outcomes. His talk pointed out the concept of cellular heterogeneity of bone marrow stromal cells (BMSCs) and presence of committed adipogenic and osteogenic progenitors besides multipotent BMSCs, which contribute to the interaction between bone and fat mass and cellular changes in the bone marrow microenvironment (13). In order to understand the mechanisms underlying age- and osteoporosis-related changes in bone formation, his laboratory discovered several secreted factors derived from BMSCs [e.g., Delta Like Non-Canonical Notch Ligand 1(DLK1), Legumain (LGMN), Cell Migration Inducing Hyaluronidase 1(KIAA1199)], which play a key role in stem cell differentiation and provide a link between bone biology and whole body energy metabolism (14–17). At the end of his talk, Dr. Kassem presented data on human BMSCs isolated from obese subjects demonstrating a hypermetabolic and accelerated senescent phenotype in comparison to lean-derived BMSCs. Dr. Kassem’s work provides important insights into metabolic changes occurring in the bone marrow microenvironment and its relation to higher bone fragility in metabolic diseases (18, 19). These data will contribute to the explanation of the obesity paradox in bone biology, as obese subjects are characterized with unchanged or increased bone mass but high rates of bone fragility.
BMAT and Bone/Bone Marrow Diseases
The second keynote lecture was given by Dr. Mara Riminucci (Sapienza University of Rome) and moderated by Dr. Ling Qin (University of Pennsylvania). The main focus of this talk was the role played by BMAT in bone and disease. She started by defining bone marrow adipocytes (BMAds), referring to Tavassoli’s definition, as recognized large cells with a single lipid droplet (20) and positive for markers such as Perilipin-1 and Adiponectin. She emphasized that both morphology and marker expression are crucial to recognizing BMAds. In terms of origin, BMAds appear in perinatal/post-natal life. The development of the “yellow marrow” (to distinguish from the hematopoietic “red marrow”) is a complex phenomenon tightly regulated by multiple genetic, developmental, and functional factors (2). Ex vivo transplantation studies have revealed a common progenitor for both BMAds and osteogenic cells that resides in the bone marrow stromal compartment (21–24). The fate choice of this common progenitor plays a crucial role in bone physiology and health. Functionally, one of the roles of BMAT is to fill the marrow space: after birth in fact, hematopoietic bone marrow becomes progressively filled with adipocytes among hematopoietic cells. However, BMA is highly flexible and reversible. Besides the space filling function, BMAT contributes to systemic metabolism (25), supports hematopoiesis (26), and modulates cell differentiation within the bone marrow stroma (27). Moreover, based on their localization as perivascular cells, BMAds regulate the sinusoid caliber and distribution of marrow blood flow (28).
The second part of the lecture focused on the role of BMAT in the context of human pathology. BMAT is, in fact, involved in different pathologies of the bone and bone marrow, including osteoporosis, bone fragility, hematological tumors, bone cancer metastasis, and some genetic disorders involving the skeleton (29, 30). On the other hand, BMAT may have its own tissue-specific pathology such as lipoma and hibernoma (31, 32).
Metabolic Function of Bone Marrow Adipose Tissue
The third keynote lecture was provided by Dr. William Cawthorn (University of Edinburgh) and moderated by Dr. William Ferris (Stellenbosch University). The presentation focused mainly on BMAT’s impact on metabolic and skeletal homeostasis within bone marrow. Dr. Cawthorn began his lecture by introducing subtypes of adipose tissue, their location, and structure. As extensive research has been done to characterize white adipose tissue (WAT) and brown adipose tissue (BAT), the talk focused on their metabolic functions, covering the processes of energy storage and energy release within WAT, as well as thermogenesis in BAT. In contrast with WAT and BAT, little is known about BMAT metabolic characteristics and its systemic function. As a leading expert in the study of BMAT, Dr Cawthorn, presented a detailed description of lipid content properties and metabolism (e.g., lipogenesis and lipolysis) in BMAT from different skeletal sites. Furthermore, his research group conducted transcriptional characterization comparing BMAT and WAT using microarray analysis. The results demonstrated that BMAT is less responsive to insulin compared to WAT (33). These findings were tested by PET/CT scan, which showed a lack of insulin-stimulated glucose uptake in BMAT (distal tibia), concluding that BMAT is insulin resistant (33). Additionally, Dr Cawthorn’s group demonstrated that BMAT is not cold responsive, as it does not increase glucose uptake during cold exposure (33, 34). Another finding, suggested that BMAT glucose uptake is able to influence systemic glucose homeostasis (33, 35). This lecture concluded with the statement that BMAT is a metabolically distinct fat depot with importance in numerous clinical conditions.
Workshops on Bone Marrow Adiposity
Standard Nomenclature, Abbreviation, and Units for the Analysis of Bone Marrow Adiposity
The first workshop entitled “Standard nomenclature, abbreviations and units for the analysis of bone marrow adipose tissue” was given by Dr. Nathalie Bravenboer (University Medical Center) and moderated by Dr. Stefanie Lucas (Université du Littoral Côte d’Opale). Dr. Bravenboer started with some historical data of the discovery of the bone marrow space and role in red blood cell development (36). The first part of her talk can be summarized with the mission of the nomenclature working group: “Our purpose is not to encourage or discourage the use of abbreviations and symbols but to ensure that the same ones are used by everybody” (10). Dr. Bravenboer listed some consensus notes: first, BMAd is the adopted abbreviation for the bone marrow adipocytes; second, BMA can be considered as a tissue, and third, anatomical location is important to give reference due to documented differences in sub-depots (e.g., proximal versus distal in the mouse tibia). The last part of this workshop was dedicated to a description of the methodologies for the analysis of BMAT including histomorphometry, Magnetic Resonance Imaging (MRI), Magnetic Resonance Spectroscopy (MRS), and Computed Tomography (CT) analysis, along with a full list of the parameters adopted to define BMAT while using each of those techniques. A detailed list of all the nomenclature and methodologies recommendations can be found in the two consensus papers published in 2021 (9, 10).
Skill Development on Histology
The second skill development workshop featured an invited talk by Dr. Erica Scheller (Washington University in St. Louis) covering histology of BMAT and was moderated by Dr. Michaela Tencerova (Institute of Physiology of the Czech Academy of Sciences). Dr. Scheller began the session outlining the potential uses of histology to study BMAds, including cell number, size, localization, molecular analyses, and lineage tracing, with a focus on the importance of technical considerations decided a priori. While the necessary considerations vary across specific histologic techniques, some factors of notable importance were generally applicable. Consistency in region of interest (ROI), analysis of a large and representative ROI, sufficient sample size (e.g., 100-300 adipocytes per sample), and the use of controls are critical to successful and reproducible histology. Hematoxylin and eosin staining of formalin-fixed paraffin-embedded tissue is ideal for investigating cell number and size (9, 37, 38). Investigation of localization or co-localization requires the usage of immunostaining methods, including both immunohistochemistry, utilizing a single antibody, and immunofluorescence, enabling multi-antibody staining (39, 40). Antibodies must be validated and optimized through positive and negative controls, including omission of the secondary antibody and tissue from genetic knockouts (41). Molecular analyses and BMAd-specific protein expression can be difficult to assess using immunostaining alone and may require lineage tracing or reporter lines (42). Despite its limitations, histology is a powerful tool for the study of BMAds and can be highly informative when care is taken to ensure rigor and reproducibility through the employment of appropriate controls and technical considerations. In summary, this workshop was an excellent introduction to the histology of BMAds for students and researchers new to the technique and will enable the usage of histological best practices.
Standard Imaging Methods in the Assessment of Bone Marrow Adipose Tissue
Dr. Dimitrios Karampinos (Technical University of Munich) delivered the third BMAT workshop: “Standard imaging methods in the assessment of bone marrow adipose tissue” that was moderated by Dr. Greet Kerckhofs (Katholieke Universiteit Leuven). Dr. Karampinos provided an extensive overview of the imaging techniques in use to assess both BMAT and bone properties, outlining the difficulties to explore both with the same imaging technique. He explained the concept of Magnetic Resonance (MR) contrast mechanisms to study fat, the T1-weighted imaging and chemical shift encoding resulting in a proton density fat fraction (PDFF). The PDFF has been used to demonstrate the fat fraction increases with aging (43, 44) and is higher in patients with osteoporosis (45). To explore in more depth the composition of fatty acids in BMAT, unsaturated and saturated fat can be measured. Data shows that saturated fats are lower in patients with fractures and/or diabetes (46), although this remains a challenging measurement especially in regions with more hematopoietic marrow (47). Finally, the newest development in BMAT imaging, i.e. measuring lipid droplet size using MRI techniques, was discussed (48). This talk was extremely useful to improve the understanding of in vivo measurement of BMAT.
Career Development Workshops
From Scientific Writing to Getting Your Paper Published
The first Career Development Workshop was given by Dr. Jonathan Tobias (University of Bristol) and moderated by Dr. Claire Edwards (University of Oxford). As a Specialty Chief Editor of the Bone Research Section in Frontiers in Endocrinology, Dr. Tobias focused on aspects of writing a scientific paper and going through the publication process. This talk covered the basic paper and journal types, reviewed the paper preparation process (e.g., paper structure and content), and the process of choosing appropriate journals for submission. Moreover, Dr. Tobias discussed the peer review process, covered the types of peer review and emphasized the importance of this process to improve the quality of publication. He also provided guidelines for responding to reviewers and tips on “Surviving the Peer Review” process, which was highly useful and informative for young researchers who are currently undergoing these processes with their first publications.
Academic Career Progression
The academic career progression talk was given by Dr. Michaela Reagan (Maine Medical Center Research/Tufts University and was moderated by Dr. Annegreet Veldhuis (Leiden University Medical Center). During the Academic Career Progression workshop, Dr. Reagan first took us with her on her academic journey and highlighted the importance of giving talks and meeting people during conferences to obtain new positions and advance your career. She shared her motivation to pursue an academic career and her keys to success, including diversification, finding a good mentor, considering work life balance, and personal traits like resiliency, open-mindedness, and most importantly, enjoying your work! Common mistakes were also discussed, such as being too optimistic, perfectionism, never saying no, and imposter syndrome. She explained the different academic career paths (tenure track, non- tenure track, or adjunct faculty) and highlighted useful grant options in the USA (e.g., NIH F31, F99/K00, NIH F32, DoD Postdoc Fellowship, or other Foundations and society). All in all, this workshop helped to gain more insight into the academic career progression and get some very useful advice.
How I Write a Grant
The career development workshop titled “How I write a grant” was given by Dr. Christopher Hernandez (Cornell University) and moderated by Dr. Julien Paccou (Lille University Hospital). Dr. Hernandez began the session detailing the importance of writing clarity in not only increasing the chances of being awarded the grant, but also in receiving feedback that will make the next grant better in the event it is not awarded. To this end, providing a strong framework, directly stating answers to review criteria, and using definitive verbiage (e.g., determine, establish, etc.) in your specific aims can improve the likelihood of receiving funding. Another key aspect of writing a grant is striking a balance between the big picture and technical details. Consideration of varying backgrounds of potential reviewers and the circumstances the grant will be read in (e.g., after hours, weekends), as well as developing skills in science communication can be extremely helpful. Numerous resources are available for additional help, including seminars, workshops, and published workbooks (49).
How to Start a Collaboration
This career development workshop continued with an invited talk by Dr. Stavroula Kousteni (Columbia University Irving Medical Center) on how to start a collaboration and was moderated by Dr. Bram van der Eerden (Erasmus University Rotterdam). This workshop was led in a very interactive format. Dr. Kousteni began by directly asking the participants what are the main aspects/points that you look for in a new collaborator (e.g., expertise, new methodology, clinical samples, animal models) as it can help to refine your search for the best collaborator. She also mentioned it is important to set up the rules of the collaboration at the beginning in order to avoid any miscommunication and delay progress of the project. Another trick to establishing successful collaborations is to find a collaborator with a similar personality. In addition, this workshop summarized several positive and negative aspects of starting new collaborations and left us with several tips on what is crucial in this important part of our scientific work.
A Path to the Science of the HeART
The career development talk entitled “A path to the science of the HeART” was given by Dr. Ana Silva and was moderated by Dr. Rossella Labella (Columbia University). Dr. Silva shared her personal transition from postdoc in academia to a freelancer position as a scientific illustrator. Like the majority of early-stage investigators in academia, she was considering becoming a Principal Investigator as her first choice. She was excluding other career options for three main reasons: ego, expectation, and fear. With the help of the MIND (Making Informed Career Decisions) program at the Gladstone Institute she decided to mix her two passions, science and illustration, and to pursue an alternative career path as a scientific illustrator. This led to the opening of her own business in Portugal. She concluded the workshop with several pros and cons of being a freelancer worker.
Learning from Failure in Life and Research
The last talk of the BMAS Summer School 2021, was given by Dr. Jennifer Heemstra (Emory University) and moderated by Dr. Erica Scheller (Washington University in St. Louis). The main goal of this talk was to share the tremendous lessons that Dr. Heemstra has learned from her scientific and life experiences. She first invited the audience to reflect on their own challenges and lessons in their career path. She began with a first lesson: your success is determined by what you do when everything is going wrong; and added that our life is the most interesting experiment that we get to run. She reminded the audience that there will be times in research and in life where things do not go as planned. It is true that these challenges and failures are frustrating, but they can also be an opportunity for us to learn and grow. She shared the many challenges she has faced in her career, including being advised not to pursue a scientific career by one of her high school academic advisors, her participation in Science Olympiad, challenging herself with the organic course chemistry where she discovered her passion for chemistry, losing her father and one of her best friends, becoming pregnant during her first week of postdoctoral training, and not passing the tenure votes. Dr. Heemstra reflected on not receiving tenure as one of the worst times of her life because she felt that she disappointed both her family and lab members. Through all of these challenges she discovered that she is stronger than she thought.
Finally, we should learn to enjoy what we are doing and do it with passion. Discovering the passion is essential and fulfilling. However, we should develop our passion rather than follow it. Dr. Heemstra ended the talk by encouraging the audience to take a step out of their comfort zone every day to challenge our capabilities. One lesson we learned from this talk: what might be your biggest failure can empower you to the most life-changing experience in your whole life.
Poster Sessions
Poster sessions consisted of a combination of pre-recorded 3-minute video presentations and a live discussion over Zoom in individual rooms. Abstracts covered a wide range of topics (Figure 3) and were divided into three categories, one per day of the summer school: 1) BMAT and stem cells, 2) BMAT, aging and cancer, and 3) BMAT and metabolic diseases. A detailed list of poster presenters and abstract titles is available in Table 1, while abstracts with consent for publication are available online (12).

Figure 3 Word cloud of the abstract keywords. The keyword size correlates with the number of times that keyword was used in the abstracts. The analysis was carried out with WordCloud package in R.
Awards
All the abstracts were evaluated from the scientific board of the BMAS in a blinded mode, each abstract was evaluated by three scientific board members and the final score was the sum of the three different evaluations. For abstract evaluation, the following criteria were considered: effectiveness of background and hypothesis, novelty of the data, clearness of communication, and overall quality of writing. The abstract that got the highest score was entitled Glucocorticoid receptor in bone marrow adipocytes is not required for expansion during calorie restriction and presented by Dr. Rebecca L. Schill from University of Michigan, USA.
In addition, the organizing committee of the summer school gave awards for the best poster presentation and for the most active participant. The former was assigned to Laura Trainor from Medical Research Institute, Adelaide, Australia and the latter to Husam Bensreti from Augusta University, Georgia, USA.
Concluding Remarks and Perspectives
Our understanding of BMA’s role and function in health and disease has grown considerably in the last years. Since 2015, an annual meeting on BMA has been organized with the aim to help collaborative research in the field and promote knowledge, which was accomplished with growing number of BMAS members and experts. In 2021, BMAS launched the first edition of the BMAS Summer School, an event organized by young scientists and addressed to trainees and junior investigators interested in the BMA field. Many biological questions remain to be addressed about the origin, development, and physiopathology of BMA, supporting the need to raise a young generation of scientists to the study of BMA. For instance, do BMAds share a common progenitor with osteoblasts? Why does BMAT develop? Do BMAds have only a filling function in bone marrow? Why do BMAds increase in conditions such as osteoporosis? Why do BMAds decrease with metastatic invasion of the bone marrow? Are there transcription factors and/or cell surface markers that are specific to BMAds and not other adipocytes? Will we be able to routinely measure BMAT in patients? Will it be possible in future to use BMAT assessment as diagnostic or prognostic tool?
BMAS Summer School 2021 was a success not only for the productive discussion coming from the insightful lectures of the speakers and the vibrant community of attendees, but also for bringing in scientists from fields other than BMA, and for the creation of a young investigator working group, the Next Generation BMAS. The main aim of the group will be to organize the biannual summer school, and other events like virtual happy hours, data discussion series, and “meet the professor” events, that will be included in the BMA meetings.
Author Contributions
RL and BP served as co-chairs of the BMAS SS 2021 meeting, and S-LL, VA, RS, MT, and AV as members of the organizing committee. BP collected and analyzed metadata. RL, S-LL, VA, RS, MT, AV, and BP wrote and revised the manuscript. All authors approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
BMAS Summer School 2021 was supported by Frontiers and the BMAS. Technical support was provided by Joey Daoud from NewTerritory Media. The organizers would like to thank all the invited speakers and the attendees.
References
1. Tavassoli M. Ultrastructural Development of Bone Marrow Adipose Cell. Cells Tissues Organs (1976) 94(1):65–77. doi: 10.1159/000144545
2. Neumann E. The Law of Distribution of Yellow and Red Marrow in the Bones of the Extremities. Cent J Med Sci (1882) 20:321–3.
3. Piney A. The Anatomy of the Bone Marrow: With Special Reference to the Distribution of the Red Marrow. Br Med J (1922) 2(3226):792–5.
4. Hardouin P, Marie PJ, Rosen CJ. New Insights Into Bone Marrow Adipocytes: Report From the First European Meeting on Bone Marrow Adiposity (BMA 2015). Bone (2016) 93:212–5. doi: 10.1016/j.bone.2015.11.013
5. van der Eerden B, van Wijnen A. Meeting Report of the 2016 Bone Marrow Adiposity Meeting. Adipocyte (2017) 6(4):304–13. doi: 10.1080/21623945.2017.1313374
6. Corsi A, Palmisano B, Tratwal J, Riminucci M, Naveiras O. Brief Report From the 3rd International Meeting on Bone Marrow Adiposity (BMA 2017). Front Endocrinol (Lausanne) (2019) 10. doi: 10.3389/fendo.2019.00336
7. Penel G, Kerckhofs G, Chauveau C. Brief Report From the 4th International Meeting on Bone Marrow Adiposity (BMA2018). Front Endocrinol (Lausanne) (2019) 10:691. doi: 10.3389/fendo.2019.00691
8. Scheller EL, McGee-Lawrence ME, Lecka-Czernik B. Report From the 6th International Meeting on Bone Marrow Adiposity (BMA2020). Front Endocrinol (Lausanne) (2021) 12:712088. doi: 10.3389/fendo.2021.712088
9. Tratwal J, Labella R, Bravenboer N, Kerckhofs G, Douni E, Scheller EL, et al. Reporting Guidelines, Review of Methodological Standards, and Challenges Toward Harmonization in Bone Marrow Adiposity Research. Report of the Methodologies Working Group of the International Bone Marrow Adiposity Society. Front Endocrinol (Lausanne) (2020) 11. doi: 10.3389/fendo.2020.00065
10. Bravenboer N, Bredella MA, Chauveau C, Corsi A, Douni E, Ferris WF, et al. Standardised Nomenclature, Abbreviations, and Units for the Study of Bone Marrow Adiposity: Report of the Nomenclature Working Group of the International Bone Marrow Adiposity Society. Front Endocrinol (Lausanne) (2020) 10. doi: 10.3389/fendo.2019.00923
11. Lucas S, Tencerova M, von der Weid B, Andersen TL, Attané C, Behler-Janbeck F, et al. Guidelines for Biobanking of Bone Marrow Adipose Tissue and Related Cell Types: Report of the Biobanking Working Group of the International Bone Marrow Adiposity Society. Front Endocrinol (Lausanne) (2021) 12. doi: 10.3389/fendo.2021.744527
12. Palmisano B, Labella R, Avilkina V, Little S, Sarkis R, Tencerova M, et al. 1st Bone Marrow Adiposity Society (Bmas) Summer School. Frontiers Media SA (2021).
13. Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the Presence of Independent Pre-Osteoblastic and Pre-Adipocytic Cell Populations in Bone Marrow-Derived Mesenchymal Stem Cells. Bone (2008) 43(1):32–9. doi: 10.1016/j.bone.2008.03.011
14. Kristensen LP, Chen L, Nielsen MO, Qanie DW, Kratchmarova I, Kassem M, et al. Temporal Profiling and Pulsed SILAC Labeling Identify Novel Secreted Proteins During Ex Vivo Osteoblast Differentiation of Human Stromal Stem Cells. Mol Cell Proteomics (2012) 11(10):989–1007. doi: 10.1074/mcp.M111.012138
15. Abdallah BM, Ditzel N, Laborda J, Karsenty G, Kassem M. DLK1 Regulates Whole-Body Glucose Metabolism: A Negative Feedback Regulation of the Osteocalcin-Insulin Loop. Diabetes (2015) 64(9):3069–80. doi: 10.2337/db14-1642
16. Jafari A, Qanie D, Andersen TL, Zhang Y, Chen L, Postert B, et al. Legumain Regulates Differentiation Fate of Human Bone Marrow Stromal Cells and is Altered in Postmenopausal Osteoporosis. Stem Cell Rep (2017) 8(2):373–86. doi: 10.1016/j.stemcr.2017.01.003
17. Tencerova M, Kassem M. The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis. Front Endocrinol (Lausanne) (2016) 7:127. doi: 10.3389/fendo.2016.00127
18. Tencerova M, Frost M, Figeac F, Nielsen TK, Ali D, Lauterlein J-JL, et al. Obesity-Associated Hypermetabolism and Accelerated Senescence of Bone Marrow Stromal Stem Cells Suggest a Potential Mechanism for Bone Fragility. Cell Rep (2019) 27(7):2050–2062.e6. doi: 10.1016/j.celrep.2019.04.066
19. Tencerova M, Okla M, Kassem M. Insulin Signaling in Bone Marrow Adipocytes. Curr Osteoporos Rep (2019) 17(6):446–54. doi: 10.1007/s11914-019-00552-8
20. Cohen L. Bone Marrow: Structure and Function by Mehdi Tavassoli and Joseph Mendel Yoffey. Perspect Biol Med (1985) 28(3):479–81. doi: 10.1353/pbm.1985.0032
21. Chan CKF, Gulati GS, Sinha R, Weissman IL, Chang HY, Longaker MT, et al. Identification of the Human Skeletal Stem Cell Article Identification of the Human Skeletal Stem Cell. Cell (2018) 175(1):43–56.e21. doi: 10.1016/j.cell.2018.07.029
22. Pinho S, Frenette PS. Haematopoietic Stem Cell Activity and Interactions With the Niche. Nat Rev Mol Cell Biol (2019) 20(5):303–20. doi: 10.1038/s41580-019-0103-9
23. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids can Organize a Hematopoietic Microenvironment. Cell (2007) 131(2):324–36. doi: 10.1016/j.cell.2007.08.025
24. Donsante S, Palmisano B, Serafini M, Robey PG, Corsi A, Riminucci M. From Stem Cells to Bone-Forming Cells. Int J Mol Sci (2021) 22(8). doi: 10.3390/ijms22083989
25. Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone Marrow Adipose Tissue is an Endocrine Organ That Contributes. Cell Metab (2015) 20(2):368–75. doi: 10.1016/j.cmet.2014.06.003
26. Valet C, Batut A, Vauclard A, Dortignac A, Bellio M, Payrastre B, et al. Adipocyte Fatty Acid Transfer Supports Megakaryocyte Maturation. Cell Rep (2020) 32(1):107875. doi: 10.1016/j.celrep.2020.107875
27. Duan D-Y, Tang J, Tian H-T, Shi Y-Y, Jia J. Adipocyte-Secreted Microvesicle-Derived Mir-148a Regulates Adipogenic and Osteogenic Differentiation by Targeting Wnt5a/Ror2 Pathway. Life Sci (2021) 278:119548. doi: 10.1016/j.lfs.2021.119548
28. Bianco P. Bone and the Hematopoietic Niche: A Tale of Two Stem Cells. Blood (2011) 117(20):5281–8. doi: 10.1182/blood-2011-01-315069
29. Nehlin JO, Jafari A, Tencerova M, Kassem M. Aging and Lineage Allocation Changes of Bone Marrow Skeletal (Stromal) Stem Cells. Bone (2019) 123:265–73. doi: 10.1016/j.bone.2019.03.041
30. Reagan MR, Fairfield H, Rosen CJ. Bone Marrow Adipocytes: A Link Between Obesity and Bone Cancer. Cancers (Basel) (2021) 13(3):364. doi: 10.3390/cancers13030364
31. Eyzaguirre E, Liqiang W, Karla GM, Rajendra K, Alberto A, Gatalica Z. Intraosseous Lipoma. A Clinical, Radiologic, and Pathologic Study of 5 Cases. Ann Diagn Pathol (2007) 11(5):320–5. doi: 10.1016/j.anndiagpath.2006.09.006
32. Thorns C, Schardt C, Katenkamp D, Kähler C, Merz H, Feller AC. Hibernoma-Like Brown Fat in the Bone Marrow: Report of a Unique Case. Virchows Arch (2008) 452(3):343–5. doi: 10.1007/s00428-007-0559-4
33. Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, et al. Bone Marrow Adipose Tissue is a Unique Adipose Subtype With Distinct Roles in Glucose Homeostasis. Nat Commun (2020) 11(1):3097. doi: 10.1038/s41467-020-16878-2
34. Pham TT, Ivaska KK, Hannukainen JC, Virtanen KA, Lidell ME, Enerbäck S, et al. Human Bone Marrow Adipose Tissue is a Metabolically Active and Insulin-Sensitive Distinct Fat Depot. J Clin Endocrinol Metab (2020) 105(7):2300–10. doi: 10.1210/clinem/dgaa216
35. Ermetici F, Briganti S, Delnevo A, Cannaò P, Di LG, Benedini S, et al. Bone Marrow Fat Contributes to Insulin Sensitivity and Adiponectin Secretion in Premenopausal Women. Endocrine (2018) 59(2):410–8. doi: 10.1007/s12020-017-1349-7
36. Cooper B. The Origins of Bone Marrow as the Seedbed of Our Blood: From Antiquity to the Time of Osler. Baylor Univ Med Cent Proc (2011) 24(2):115–8. doi: 10.1080/08998280.2011.11928697
37. Beeve AT, Shen I, Zhang X, Magee K, Yan Y, MacEwan MR, et al. Neuroskeletal Effects of Chronic Bioelectric Nerve Stimulation in Health and Diabetes. Front Neurosci (2021) 15. doi: 10.3389/fnins.2021.632768
38. Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying Size and Number of Adipocytes in Adipose Tissue. Methods Enzymol (2014) 537:93–122. doi: 10.1016/B978-0-12-411619-1.00006-9
39. Lorenz MR, Brazill JM, Beeve AT, Shen I, Scheller EL. A Neuroskeletal Atlas: Spatial Mapping and Contextualization of Axon Subtypes Innervating the Long Bones of C3H and B6 Mice. J Bone Miner Res (2021) 36(5):1012–25. doi: 10.1002/jbmr.4273
40. Idleburg C, Lorenz MR, DeLassus EN, Scheller EL, Veis DJ. Immunostaining of Skeletal Tissues. Methods Mol Biol (2021) 2021:261–73. doi: 10.1007/978-1-0716-0989-7_15
41. Pontén F, Schwenk JM, Asplund A, Edqvist P-HD. The Human Protein Atlas as a Proteomic Resource for Biomarker Discovery. J Intern Med (2011) 270(5):428–46. doi: 10.1111/j.1365-2796.2011.02427.x
42. Craft CS, Robles H, Lorenz MR, Hilker ED, Magee KL, Andersen TL, et al. Bone Marrow Adipose Tissue Does Not Express UCP1 During Development or Adrenergic-Induced Remodeling. Sci Rep (2019) 9(1):17427. doi: 10.1038/s41598-019-54036-x
43. Griffith JF, Yeung DKW, Ma HT, Leung JCS, Kwok TCY, Leung PC. Bone Marrow Fat Content in the Elderly: A Reversal of Sex Difference Seen in Younger Subjects. J Magn Reson Imaging (2012) 36(1):225–30. doi: 10.1002/jmri.23619
44. Baum T, Rohrmeier A, Syväri J, Diefenbach MN, Franz D, Dieckmeyer M, et al. Anatomical Variation of Age-Related Changes in Vertebral Bone Marrow Composition Using Chemical Shift Encoding-Based Water–Fat Magnetic Resonance Imaging. Front Endocrinol (Lausanne) (2018) 9. doi: 10.3389/fendo.2018.00141
45. Karampinos DC, Ruschke S, Dieckmeyer M, Diefenbach M, Franz D, Gersing AS, et al. Quantitative MRI and Spectroscopy of Bone Marrow. J Magn Reson Imaging (2018) 47(2):332–53. doi: 10.1002/jmri.25769
46. Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone Marrow Fat Composition as a Novel Imaging Biomarker in Postmenopausal Women With Prevalent Fragility Fractures. J Bone Miner Res (2013) 28(8):1721–8. doi: 10.1002/jbmr.1950
47. Syväri J, Ruschke S, Dieckmeyer M, Hauner HH, Junker D, Makowski MR, et al. Estimating Vertebral Bone Marrow Fat Unsaturation Based on Short-TE STEAM MRS. Magn Reson Med (2021) 85(2):615–26. doi: 10.1002/mrm.28453
48. Weidlich D, Honecker J, Boehm C, Ruschke S, Junker D, Van AT, et al. Lipid Droplet–Size Mapping in Human Adipose Tissue Using a Clinical 3T System. Magn Reson Med (2021) 86(3):1256–70. doi: 10.1002/mrm.28755
Keywords: bone marrow adipose tissue (BMAT), bone marrow adiposity, bone marrow adipocytes, metabolism, imaging technique, BMSC – bone marrow stromal cells, histology, career development
Citation: Labella R, Little-Letsinger S, Avilkina V, Sarkis R, Tencerova M, Vlug A and Palmisano B (2022) Next Generation Bone Marrow Adiposity Researchers: Report From the 1st BMAS Summer School 2021. Front. Endocrinol. 13:879588. doi: 10.3389/fendo.2022.879588
Received: 19 February 2022; Accepted: 21 March 2022;
Published: 13 April 2022.
Edited by:
Andre Van Wijnen, University of Vermont, United StatesReviewed by:
Michaela Ruth Reagan, Maine Medical Center Research Institute, United StatesMeghan E McGee-Lawrence, Augusta University, United States
Copyright © 2022 Labella, Little-Letsinger, Avilkina, Sarkis, Tencerova, Vlug and Palmisano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biagio Palmisano, biagio.palmisano@uniroma1.it
†These authors have contributed equally to this work and share first authorship
 Rossella Labella
Rossella Labella Sarah Little-Letsinger
Sarah Little-Letsinger Viktorjia Avilkina
Viktorjia Avilkina Rita Sarkis
Rita Sarkis Michaela Tencerova
Michaela Tencerova Annegreet Vlug
Annegreet Vlug Biagio Palmisano
Biagio Palmisano