No CrossRef data available.
Article contents
Shinarumpite, a new cobalt uranyl sulfate mineral from the Scenic mine, San Juan County, Utah, USA, structurally related to leydetite
Published online by Cambridge University Press: 28 November 2022
Abstract
The new mineral shinarumpite (IMA2021-105), [Co(H2O)6][(UO2)(SO4)2(H2O)]⋅4H2O, was found in the Scenic mine on Fry Mesa, White Canyon district, San Juan County, Utah, USA, where it occurs as a secondary phase on granular quartz matrix in association with gypsum, deliensite, Co-rich rietveldite, scenicite, shumwayite and sulfur. Shinarumpite crystals are transparent, yellow, blades or prisms, up to 1 mm in length. The mineral has white streak, vitreous lustre and is nonfluorescent. It is brittle with irregular, curved fracture. The Mohs hardness is ~2½ and it has a perfect {100} cleavage. The density is 2.58(2) g⋅cm–3. Optically, the mineral is biaxial (–) with α = 1.515(2), β = 1.526(2), γ = 1.529(2) (white light); 2V = 55(1)°; extreme r < v dispersion; orientation: Z = b, X ^ a = 30° in obtuse β; pleochroism: X = very pale yellow, Y = pale yellow, Z = light yellow; X < Y < Z. The Raman spectrum exhibits bands consistent with UO22+, SO42– and O–H. Electron microprobe analysis provided the empirical formula [(Co0.51Ni0.28Fe0.21)Σ1.00(H2O)6][(UO2)(SO4)2(H2O)]⋅4H2O. The five strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 10.37(100)(200), 5.73(43)(111), 5.20(70)(400, 202, 211), 4.70(31)($\bar{3}$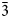 11) and 3.326(30)(213, 021). Shinarumpite is monoclinic, P21/c, a = 21.0549(15), b = 6.8708(5), c = 12.9106(5), β = 96.678(7)°, V = 1885.03(17) Å3 and Z = 4. In the structure of shinarumpite (R1 = 0.0336 for 2623 I > 2σI), linkages of pentagonal bipyramids and tetrahedra form an infinite [(UO2)(SO4)2(H2O)]2– sheet. Isolated Co(H2O)6 octahedra and H2O groups occupy the interlayer region linking the sheets via an extensive system of hydrogen bonds. The structure of shinarumpite is very similar to that of leydetite. Uranyl sulfate structural unit types are discussed with respect to frequency and charge deficiency per anion (CDA).
11) and 3.326(30)(213, 021). Shinarumpite is monoclinic, P21/c, a = 21.0549(15), b = 6.8708(5), c = 12.9106(5), β = 96.678(7)°, V = 1885.03(17) Å3 and Z = 4. In the structure of shinarumpite (R1 = 0.0336 for 2623 I > 2σI), linkages of pentagonal bipyramids and tetrahedra form an infinite [(UO2)(SO4)2(H2O)]2– sheet. Isolated Co(H2O)6 octahedra and H2O groups occupy the interlayer region linking the sheets via an extensive system of hydrogen bonds. The structure of shinarumpite is very similar to that of leydetite. Uranyl sulfate structural unit types are discussed with respect to frequency and charge deficiency per anion (CDA).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland.
Footnotes
Associate Editor: Michael Rumsey




