Abstract
We have prepared a candidate biocompatible construct for skin wound healing based on electrospun polycaprolactone (PCL) nanofibrous membranes. The membrane material was loaded either with L-arginine or with alaptide, or with a mixture of both bioactive components. Alaptide is a spirocyclic synthetic dipeptide, an analogue of melanocyte-stimulating hormone release-inhibiting factor. L-arginine is an amino acid with a basic guanidine side chain. It is a direct precursor of nitric oxide, which plays a pivotal role in skin repair. The presence and the distribution of the additives were proved with high-performance liquid chromatography, Fourier-transform infrared spectroscopy and Raman spectroscopy. The influence of L-arginine and alaptide on the morphology of the membrane was characterized using scanning electron microscopy. No statistically significant correlation between fiber diameter and drug concentration was observed. The membranes were then tested in vitro for their cytotoxicity, using primary human dermal fibroblasts, in order to obtain the optimal concentrations of the additives for in vivo tests in a rat model. The membranes with the highest concentration of L-arginine (10 wt. %) proved to be cytotoxic. The membranes with alaptide in concentrations from 0.1 to 2.5 wt.%, and with the other L-arginine concentrations (1 and 5 wt.%), did not show high toxicity. In addition, there was no observed improvement in cell proliferation on the membranes. The in vivo experiments revealed that membranes with 1.5 wt.% of alaptide or with 1.5 wt.% of alaptide in combination with 5 wt.% of L-arginine markedly accelerated the healing of skin incisions, and particularly the healing of skin burns, i.e. wounds of relatively large extent. These results indicate that our newly-developed nanofibrous membranes are promising for treating wounds with large damaged areas, where a supporting material is needed.
Export citation and abstract BibTeX RIS
1. Introduction
Wound healing is a complex process, which is usually divided into three overlapping stages—(1) hemostasis and inflammation, (2) proliferation, and (3) remodeling [1]. Although the skin is able in due course to repair most wounds by itself, there are a number of adverse factors, including potential infection (sepsis) and contamination of the wound with foreign bodies [2]. These factors may result in a prolonged healing period, and possibly in the formation of a chronic wound. Such a wound requires the use of antibiotics, which can produce negative side effects, e.g. sleep disorders, headaches and diarrhea [3]. Localized drug delivery to the wound is therefore being studied as an alternative to generalized drug application. It has been known for a very long time that the use of an appropriate wound dressing can significantly improve the wound healing process. Moreover, recent studies have shown that wounds covered with moisture-retentive dressings heal faster than wounds left open to the air or covered by traditional wound dressings, e.g. lint, gauzes and cotton wools [4]. Nowadays, wound dressings are mostly based on synthetic polymers that can effectively adjust wound moisture by absorbing the excess exudate, and can provide sufficient oxygen permeability [3, 5]. In addition, polymeric wound dressings can be conveniently functionalized with a wide range of biomolecules and drugs [6, 7]. A promising group of such biomolecules are short peptides. Peptides are interesting organic compounds which can be used for a variety of applications in bio-nanotechnology [8]. More specifically, short peptides are a topic of constantly growing interest in medical applications due to their positive effects on wound healing in terms of their antibacterial properties and acceleration of the healing process [9]. In our study, the dipeptide alaptide and the amino acid L-arginine were used in the tests. Both biomolecules have good potential in the wound healing process, and are described in separate sections. They were incorporated into electrospun nanofibrous poly ( -caprolactone) membranes as a candidate/model wound dressing.
-caprolactone) membranes as a candidate/model wound dressing.
Alaptide, i.e. 8(S)-methyl-6,9-diazaspiro [4, 5] dekan-7,10-dione (see figure 1), is a spirocyclic synthetic dipeptide which was discovered in the 1980 s by Czech chemists Šturc and Kasafírek [10]. It was developed as an analogue of the melanocyte-stimulating hormone release-inhibiting factor (MIF). Alaptide was selected from among a series of other spirocyclic derivatives as the most advantageous MIF analogue from the point of view of its enzymatic stability and due to its pharmacodynamical profile [10, 11]. Alaptide can be classified as nootropic; for example, it was experimentally found to have an effect on the short-term memory and the learning abilities of rats and mice [12, 13]. However, in the current study, alaptide was chosen mainly for its favorable results in dermatological experiments: a considerable number of tests have shown that alaptide can have a positive influence on epidermal regeneration. In vivo experiments, performed on domestic pigs, rats and mice, proved that alaptide accelerated skin regeneration and the curing of experimental skin injuries [14]. Moreover, very low acute toxicity was observed in rats and mice, i.e. a 1 g/1 kg dose caused only 20% mortality of female rats [12, 13]. This low toxicity can reduce one of the major disadvantages of transdermal drugs—the possibility of local skin irritation and allergization. Alaptide is currently successfully used as a veterinary 1% hydrophilic ointment under the name ALAPTID® (Bioveta, Czech Republic) for treating warm-blooded animals in order to cure local injuries such as burns, frost-bite, bedsores, etc [15]. The release of alaptide from other formulations, e.g. from cellulose-based hydrogels, has also been studied [16].
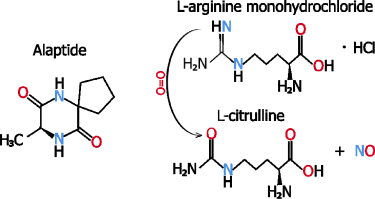
Figure 1. Structure of (S)-alaptide (left) and hydroxylation of L-arginine to L-citrulline with release of nitric oxide.
Download figure:
Standard image High-resolution imageL-Arginine is a non-essential amino acid with a positively charged basic side chain. It plays an important role during the wound healing process. Its positive effect on wound healing is mainly attributed to the release of nitric oxide and multifunctional free radicals [17, 18]. Nitric oxide plays a pivotal role in skin repair. It influences the activities of macrophages, fibroblasts and keratinocytes during wound healing. L-arginine and its metabolism products are required for tissue repair of acute and chronic wounds. They stimulate the release of growth hormone, and also the release of insulin-like growth factor I (IGF-1), both of which can improve wound healing [19]. The body does not provide sufficient amounts of L-arginine for metabolic needs during the healing process. This amino acid is synthetized in healthy humans, but additional L-arginine is needed after injury. A comprehensive review on the L-arginine metabolism and healing has been written by Stechmiller et al [18]. Modifying nanofibers with L-arginine has been dealt with in only a small number of papers. Subramaniyan et al coated polyurethane nanofibers with L-arginine for biological in vitro testing [20]. Other authors have modified the surface of a lignin or cellulose nanofiber gel with L-arginine for wound healing [21, 22].
In our study, both additives were incorporated, first separately and later in combination, into nanofibers, and were then biologically tested. The reason for combining these two biomolecules was to test their possible synergetic effect on accelerating the wound healing process.
Nanofibrous wound dressings were produced with the use of electrospinning technology, which is a versatile method for producing biologically functional fibrous structures for further applications in tissue regeneration as wound dressings, drug delivery systems and tissue scaffolds [23]. A number of studies have reported successful incorporation of various drugs into electrospun nanofibers [24, 25]. It was also proven that the high voltage applied in the electrospinning process had no effect on the bioactivity of drugs and biomolecules [26]. Nowadays, a number of strategies are available, such as emulsion electrospinning, blend electrospinning and co-axial electrospinning in order to functionalize electrospun nanofibers with bioactive agents [27, 28]. Electrospinning is suitable for a wide range of polymers. However, it is a great challenge to find an applicable polymer that combines biocompatibility, biodegradability and non-toxicity [26]. One option is to use natural polymers, such as polysaccharides or proteins. However, there are variabilities in the characteristics of natural polymers that introduce some difficulties in terms of the wide range of molecular weight distributions for each batch. Aliphatic polyesters, such as polylactic or polyglycolic acid, polycaprolactone, etc, are obvious candidates for our purposes.
In our study, poly- -caprolactone (PCL), a linear hydrophobic aliphatic semi-crystalline polymer, was used for electrospinning to produce a nanofibrous matrix. PCL has been attracting great attention in tissue engineering and in controlled drug delivery due to its desirable characteristics, such as biodegradability and cytocompatibility [29, 30]. On the one hand, it is known that the use of hydrophobic polymers as drug delivery devices can sustain and control drug release for relatively long time periods [31]. On the other hand, the hydrophobicity of PCL can have a negative effect on the cell adhesion and proliferation, which in turn inhibits the wound healing process [32]. Low glass transition and low melting temperatures can also be considered as major disadvantages of PCL—e.g. in the case of thermal sterilization. Its low melting temperature prevents PCL being processed like conventional thermoplastic polymers, e.g. by autoclaving [33]. Nevertheless, ethylene oxide (EtO) sterilization is suitable for most low-melting polymers. The EtO has to be given with enough time to dissipate from the material after the sterilization process. Sterilization by immersion in ethanol (EtOH) is also often used as an inexpensive, quick, low-temperature technique, although it involves biochemical and morphological changes to the scaffold [34].
-caprolactone (PCL), a linear hydrophobic aliphatic semi-crystalline polymer, was used for electrospinning to produce a nanofibrous matrix. PCL has been attracting great attention in tissue engineering and in controlled drug delivery due to its desirable characteristics, such as biodegradability and cytocompatibility [29, 30]. On the one hand, it is known that the use of hydrophobic polymers as drug delivery devices can sustain and control drug release for relatively long time periods [31]. On the other hand, the hydrophobicity of PCL can have a negative effect on the cell adhesion and proliferation, which in turn inhibits the wound healing process [32]. Low glass transition and low melting temperatures can also be considered as major disadvantages of PCL—e.g. in the case of thermal sterilization. Its low melting temperature prevents PCL being processed like conventional thermoplastic polymers, e.g. by autoclaving [33]. Nevertheless, ethylene oxide (EtO) sterilization is suitable for most low-melting polymers. The EtO has to be given with enough time to dissipate from the material after the sterilization process. Sterilization by immersion in ethanol (EtOH) is also often used as an inexpensive, quick, low-temperature technique, although it involves biochemical and morphological changes to the scaffold [34].
The electrospun PCL membranes were tested for their morphology and their initial drug loadings. Sterilized samples containing both additives in various concentrations were first subjected to in vitro tests to obtain optimal non-cytotoxic loadings. On the basis of the results of in vitro tests, three concentrations for both additives and for a combination of the additives were selected for producing new materials for in vivo tests that were carried out on two different types of skin wounds (i.e. burns and incisions) on rats.
2. Materials and methods
2.1. Preparation of nanofibrous membranes
All materials were electrospun using Nanospider NS 1WS500 U (Elmarco a.s. Czech Republic). PCL (Merck, KGaA 704 105) with average molecular weight Mn 45 kg mol−1 was dissolved in a chloroform/ethanol (Penta s.r.o., Czech Republic) solvent mixture at a weight ratio of 8/2. Ethanol was used as a less volatile solvent (in comparison with chloroform) to control the evaporation rate of the solvent system.
The polymer concentration used for obtaining continuous fibrous meshes was 16 wt.%. The L-arginine-modified PCL meshes were prepared by adding 1, 5 or 10 wt.% of L-arginine monohydrochloride (Merck KGaA, Germany) in the form of a cryomilled powder. First, 0.075 wt.% of sodium lauryl sulphate (SLS) was dissolved as a stabilizer in the same solvent system mentioned above for PCL. A given amount of L-arginine was then dissolved/dispersed in this solvent system for 30 min; this was followed by 10 min of sonication to ensure the homogeneity of the L-arginine suspension. Following the addition of PCL pellets, the resulting solution was stirred constantly until the PCL had completely dissolved, whereupon it was immediately electrospun. The addition of 1 wt.% of L-arginine led to the formation of electrospinning solutions, whereas the addition of 5 and 10 wt.% gave rise to a suspension containing partly undissolved L-arginine particles. The same procedure was performed for alaptide, and later for the alaptide/L-arginine mixture. Alaptide was synthetized by the standard process by a two-step procedure [35]. In this study, we used 0.1, 1 and 2.5 wt.% of alaptide added into the PCL solution, and for membranes with the alaptide/L-arginine combination, the loading was 1.5 wt.% of alaptide and 5 wt.% of L-arginine in the PCL solution.
The concentrations of the additives mentioned throughout this article were calculated with respect to the polymer solution from which the nanofibrous membrane was spun. The concentrations of the additives in the nanofibers therefore have to be recalculated with respect to the dry content only. For example, 1 wt.% in the PCL solution means that 1 g of the drug was added to 16 g of PCL and was dissolved in 83 g of the solvent. This finally yields 6.25 wt.% of the drug in the dry PCL nanofibrous membrane. The drug concentrations recalculated per dry PCL are given in (table 1). However, these numbers are only theoretical, and would be valid only in the case of full dissolution of the additives in the solvent system and an ideal spinning procedure.
Table 1. Initial loadings of both additives measured by HPLC from PCL electrospun mats and predicted by weighing.
| L-arginine loading capacity in PCL | ||||
|---|---|---|---|---|
| Loading in solution (wt.%) | Theoretical loading in dry PCL (wt.%) | Predicted loading (mg/g) | Measured loading (mg/g) | Difference of the measured and predicted loading (%) |
| 1 | 6.25 | 58.824 | 68.197 ± 5.385 | 16 ± 0.9 |
| 5 | 31.25 | 238.095 | 299.261 ± 12.720 | 12.6 ± 0.5 |
| 10 | 62.5 | 384.615 | 474.323 ± 55.926 | 12.33 ± 1.45 |
| Alaptide loading capacity in PCL | ||||
| 0.1 | 0.625 | 6.211 | 6.355 ± 0.571 | 1.23 ± 0.9 |
| 1 | 6.25 | 58.824 | 71.776 ± 5.556 | 12.2 ± 0.94 |
| 1.5 | 9.375 | 85.714 | 101.613 ± 10.664 | 11.85 ± 1.25 |
| 2.5 | 15.625 | 135.135 | 159.271 ± 15.772 | 11.8 ± 1.16 |
For electrospinning, a positive voltage was applied to the wire (i.e. the spinning electrode) and a negative voltage was applied to the collector; the potential difference was 40 kV. The distance between the spinning electrode and the collector was 160 mm. The temperature during the experiments was 22 ± 5 °C, with relative humidity of 40 ± 5%. The fibers were analyzed by means of scanning electron microscopy (SEM, Tescan VEGA3 SB, Czech Republic), and the fiber diameters were measured (200 fibers per sample) using ImageJ software. The normality of the data was verified primarily by the Shapiro-Wilk test. Homoscedasticity was verified by the Levene test. Non-parametric analysis was employed since the assumption either of normality or of homoscedasticity was violated and, subsequently, the Mann-Whitney test was performed in the case of two-sample comparisons. Statistical significance was accepted at p ≤ 0.05.
2.2. High-performance liquid chromatography
The alaptide and L-arginine concentration in each collected aliquot, and also in the dissolved fibrous samples, was determined by high-performance liquid chromatography (HPLC). The HPLC system that was used was a Dionex Ultimate 3000 with an LPG-3400SD quaternary gradient pump, an SR-3000 solvent rack, a WPS-3000TSC autosampler, a TCC-3000SD column compartment and a DAD-3000 detector. A Phenomenex Kinetex Hilic core–shell column 150 mm in length and 4.6 mm in internal diameter was used. The aqueous component (A) of the mobile phase consisted of 5% acetonitrile in water. The organic component (B) of the mobile phase consisted of pure acetonitrile.
2.3. FTIR and Raman spectroscopy
The presence of the two additives was tested by attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) and by Raman spectroscopy. ATR-FTIR spectra (600–4000 cm−1, 128 scans, resolution 4 cm−1) were recorded on a Nicolet 6700 FTIR spectrometer with a ZnSe crystal as an ATR accessory. Linear baseline correction was performed in the OMNIC 32 program, ver. 8.2 (Thermo Scientific, USA). Fourier-transform (FT) Raman spectra (100–4000 cm−1, 1204 scans, resolution 4 cm−1) were recorded on a Bruker FT Raman (FRA 106/S, Equinox 55/S) spectrometer equipped with an Nd:YAG laser (λex = 1064 nm, Coherent, Santa Clara, USA), a quartz beam splitter and a Ge detector (cooled with liquid N2).
2.4. In vitro drug release tests
First, small nanofibrous samples with average weights of 50 ± 0.9 mg were cut from the nanofibrous layers of each material for the investigation of the release of the drug. The samples were then immersed in 5 ml of phosphate-buffered saline (PBS) solution (pH 7.4) and were incubated at 37 ± 1 °C. Small aliquots of 1 ml were extracted from the tubes at predetermined time intervals (i.e. 30 min, 1 h, 5 h and 10 h) and were replaced with fresh PBS solution in order to maintain sink conditions. The aliquots obtained were analyzed by means of HPLC. The cumulative amount of the released drug was calculated using the following equation:
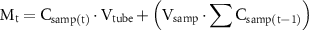
where Csamp(t) [mg l−1] is the concentration of the drug in the aliquot sample at time t, Vtube [L] is the overall volume of the tube containing the release medium (5 ml), and Vsamp [L] is the volume of the aliquot (1 ml).
2.5. Determination of drug loading
The drug load of the nanofibrous membranes was measured by HPLC. The samples with alaptide were dissolved in 4 ml of chloroform/acetonitrile (1:1) solution and were then vortexed. The obtained solution with the dissolved fibers was mixed with pure methanol 1:1 and was vortexed. Finally, 850 µl of the methanol-containing solution was mixed with 150 µl of distilled water and 3 ml of pure acetonitrile and was vortexed.
The samples with L-arginine were dissolved in 2 ml of chloroform. The resulting solution was then mixed 1:1 with 2 ml of 0.25% heptafluorobutyric acid and 0.5% formic acid in water. This procedure resulted in the creation of a separate liquid phase with diluted L-arginine. One milliliter of this separated phase was used for the HPLC analysis. The alaptide and L-arginine solutions were both filtered using a 0.22 µm nylon filter before proceeding with the HPLC analysis.
2.6. In vitro cell metabolic activity
Normal human dermal fibroblasts (NHDF, Lonza, Basel, Switzerland) were used for the cytotoxicity tests of pure and modified PCL nanofibrous membranes, because the intended utilization of this material was in the field of skin tissue engineering and regenerative medicine. The cells were cultivated under standard cultivation conditions, i.e. in an incubator with 37 °C and 5% CO2 atmosphere, and in a high glucose DMEM cultivation medium with phenol red (Gibco, ThermoFisher Scientific, Waltham, MA, USA, Cat. no.: 52100021) containing 10 vol.% of fetal bovine serum (FBS; Gibco, ThermoFisher Scientific, Waltham, MA, USA). The studied PCL membranes were cut into circular pieces 16 mm in diameter. The samples were then sterilized in an EtO atmosphere and were left in a container to prevent them being contaminated by infectious agents. The container was not airtight, in order to let the EtO dissipate for three weeks. The samples were then fixed in CellCrown holders (Scaffdex Ltd., Tampere, Finland), and were inserted into 24-well plates (TPP, Switzerland) in a sterile flow-box. The NHDF cells were seeded on the sample in a concentration of 20 000 cells/cm2 and were cultivated for 1, 3 and 7 d. After these cultivation periods, the cell mitochondrial metabolic activity was measured by an MTS assay (Promega, USA). The MTS reagent was diluted in low glucose DMEM (Sigma-Aldrich, USA, cat. no.: D2902) + 10% FBS medium without phenol red in a ratio of 1:5, according to the provider's protocol. The cells were cultivated with MTS for 2 h. The MTS-containing medium (100 μl/well) was then transferred to a 96-well plate (Greiner Bio-One GmbH, Kremsmünster, Austria), and the absorbance was measured for wavelengths 490 nm and 650 nm as a background control on a VersaMax microplate reader (Molecular Devices, San Jose, CA, USA). The results of the MTS tests were evaluated using SigmaStat (Systat, USA). The results were compared, and statistically significant differences were evaluated using the one way ANOVA test with Tukey's post-hoc test (only alaptide and only L-arginine experiments) or Dunn's post-hoc test (alaptide + L-arginine experiments). Statistical significance was accepted at p ≤ 0.05.
2.7. Fluorescence microscopy
After the 1-, 3- and 7 d cultivation periods, the NHDF cells were also fixed by 4 wt.% paraformaldehyde in PBS. The cells were then stained for cell nuclei with a DNA-binding DAPI dye, and for the actin cytoskeleton by Phalloidin conjugated with TRITC (Life Technologies, CA, US). The cells were then photographed on an IX71 Olympus microscope, equipped with a DP71 digital camera and a 10x (N.A. = 0.3) lens.
2.8. The animal model and in vivo experiments
Female albino laboratory rats (Rattus norvegicus, Wistar strain, age 2–3 months, weight 250–280 g, Velaz s.r.o., Prague, Czech Republic) were used for the in vivo tests of the healing capabilities of the PCL membranes. Two types of skin wounds were studied, namely skin incisions (15–20 mm in length) and circular skin burns (∅ 25 mm) in the dorsal area of the animals. Initially, all rats were anaesthetized through an intramuscular injection of xylazine/ketamine (16 mg/100 g of weight). Then the rats were divided into three main groups. In the control group, the animals were without any injury or healing treatment. The subsequent two groups with a specific type of injury (burns or incisions) were divided into subgroups according to the healing treatment process (untreated wound, wounds treated with PCL/alaptide nanofibers and with PCL/alaptide/L-arginine nanofibers). The burns were caused by a hot metal steel coin with a nickel coating approximately 25 mm in diameter for 5 s. The coin was heated up to 500 °C. The incisions were made using a sterile scalpel.
Based on the results of the in vitro experiments, membranes containing only 1.5 wt.% of alaptide or of a mixture of 1.5 wt.% of alaptide and 5 wt.% of L-arginine were prepared for the in vivo experiments. The wound healing process was monitored in 7 d intervals for a period of 35 d for the burns, and for a period of 21 d for the incisions. The sizes of the wound areas were determined by image analysis using ImageJ/Fiji 1.46 software (LOCI, University of Wisconsin, USA). The measured values were compared for each time interval using parametric one-way ANOVA with Tukey's post hoc test in SigmaStat software (Systat, USA). Statistical significance was accepted at p ≤ 0.05. Each group and subgroup included three animals kept in individual cages and under the same conditions of maintenance and feeding. The nanofibrous layer was fixed by Epiglu tissue adhesive (Meyer-Haake GmbH Medical Innovations, Germany) on the edges of the layer to prevent it coming into contact with the wound. All the procedures on the living animals in the course of this study were performed at the 1st Faculty of Medicine, Charles University in Prague, Czech Republic. The in vivo experiments were approved by the animal care committee of the same faculty, and were conducted in accordance with its established guidelines.
3. Results
3.1. Characterization of nanofibrous membranes
All the membranes were spun from suspensions due to the low solubility of both additives in the solvents that were used. L-arginine is soluble in water and slightly soluble in ethanol [36]. Alaptide is poorly soluble in ethanol (0.1011 g/100 ml) and also in chloroform (0.05 mg/100 ml) [15, 37]. All the layers were therefore spun mainly from suspensions, with the exception of 0.1 wt.% alaptide, which was completely dissolved in the chloroform/ethanol mixture. This mixture was used because chloroform is a good solvent of PCL. However, chloroform has a moderate dielectric constant and creates fibers with relatively large diameters. The increase in the dielectric constant with the addition of ethanol, methanol or dimethylformamide produces fibers with a lower distribution profile of the fiber diameter [38]. Addition of ethanol was chosen due to its relatively low toxicity. Another option is to use the acetic and formic acid solvent system. This system reduces the diameter of the fibers as the dielectric constant increases, but the solution is unstable in time [39].
The additives were incorporated into the fibers in the form of randomly distributed individual particles (see figure 2).
Figure 2. SEM of encapsulated particles of alaptide (A) and L-arginine (C) (highlighted in blue) in PCL nanofibers, and particles of pure alaptide (B) and L-arginine (D), for comparison. The turquoise ovals and circles indicate areas with incorporated particles. Red bars represent 50 μm.
Download figure:
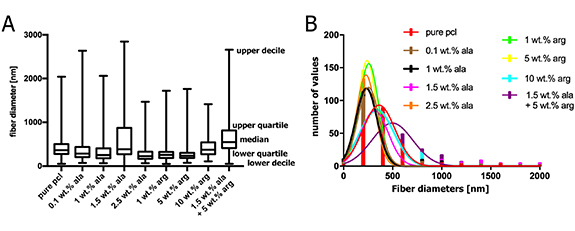
Standard image High-resolution imageWe obtained PCL meshes with two fiber-size populations, as shown in the micrographs in figure 3. This is mainly due to the use of a critical concentration of the polymer that already forms fibers without beads for a given solvent system and molecular weight distribution [40]. With increasing concentration of the polymer, or with its higher molecular weight, mono-modal but thicker fibers would be obtained [30]. The nanofibrous layers that were obtained had a wide range of fiber diameters. Only the layers with 1.5 wt.% Ala and 10 wt.% of Arg were not significantly different from the control sample (pure PCL, see figure 4).
Figure 3. SEM pictures of all membranes that were produced and tested, i.e. made of pure PCL and PCL loaded with various concentrations of L-arginine (Arg) and alaptide (Ala). Red bars represent 10 μm.
Download figure:
Standard image High-resolution imageFigure 4. (A) Box plots of fiber diameter distributions for all nanofibrous PCL-based membranes used for the experiments; data were acquired from 200 measurements for each sample type. The central line of box plots represents median, outer edges represent the first and the third quartile, the whiskers span from the first decile to the last decile. Studied samples were compared to the pure PCL control using the Mann-Whitney test, statistical significance: **** p ≤ 0.0001. (B) Histograms of the fiber diameter distributions in all studied samples.
Download figure:
Standard image High-resolution imageIt is known that the majority of monolith (matrix-type) drug delivery systems perform a "burst release". This is mainly due to some amount of the drug being trapped on (or near) the surface of the delivery carrier during the electrospinning process [41]. The fact that these particles (or molecules) have a short diffusional path results in rapid release shortly after placement in the release medium.
The drug loading values measured by HPLC were slightly different from the predicted (calculated) values. The only exception was 0.1 wt.% of alaptide, which was completely dissolved in the PCL solution (table 1). The inconsistency related to the drug loading capacity for higher loadings may have occurred due to the presence of both the dissolved drug and the undissolved drug within the nanofibrous mats. Moreover, the difference between the predicted and the actual loading could have been caused by the polarity of the drug molecule which may, in turn, have led to the rearrangement of the particles in the polymer solution as a result of the presence of an electrostatic field during the electrospinning process [42]. Thus, it appears that the drug-containing fraction of the precursor PCL solution was spun preferentially.
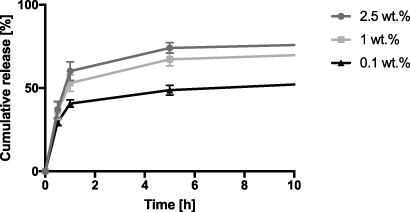
The cumulative amount of alaptide that was released is plotted as a function of time for the first 10 h (see figure 5). We can see that the first 60% is released within the first 1 h, which corresponds with reports in the literature [31]. However, this is not true for 0.1 wt.% of alaptide, because it was fully dissolved in the current solvent system. The drug is therefore present in the form of individual molecules, and its release is driven by classical diffusion in a polymer [43].
Figure 5. Release kinetics of alaptide with different concentrations for the first 10 h.
Download figure:
Standard image High-resolution imageThe same method for investigating the drug release was also provided for L-arginine. Nevertheless, the total released amount of arginine decreased along with time. This was probably due to the gradual degradation of L-arginine to L-citrulline in the release medium caused by the change in pH (see figure 1). This in turn distorted the HPLC measurement. We therefore measured the change in pH with time. The measurements of the change in the pH of the water with immersed PCL membranes with various amounts of L-arginine indicate that the release of L-arginine from the membrane significantly lowers the pH of the water within a period of 2–3 h (figure 6).
Figure 6. Change in the pH of demineralized water after incorporating the PCL membrane with various amounts of L-arginine. The first measurement was made at t = 30 min.
Download figure:
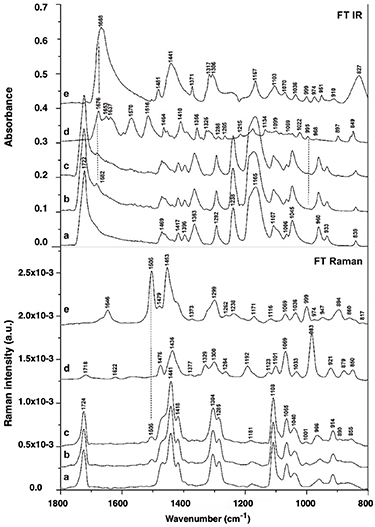
Standard image High-resolution imageFourier-transform infrared spectroscopy (FTIR) and Fourier-transform Raman spectroscopy (FT Raman) were used for evaluating the presence of both additives in the nanofibrous membranes. FTIR and FT Raman spectra in the 1800–800 cm−1 region of pure PCL nanofibers, L-arginine, alaptide and PCL membrane modified with alaptide and alaptide/L-arginine mixture are presented in figure 7.
Figure 7. FTIR and FT Raman spectra of pure PCL nanofibrous membranes (a), PCL membranes modified with alaptide (b) or with an alaptide/L-arginine mixture (c); free L-arginine (d) and free alaptide (e).
Download figure:
Standard image High-resolution imageVibrational bands of pure PCL nanofibrous membrane (a) predominate in the spectra of this membrane after modification with alaptide (b) or with alaptide/l-arginine mixture (c). These bands corresponded with asymmetric or symmetric stretching and deformational vibrations of the backbone and the functional groups in PCL polymer. The intense band of C = O stretching vibration at 1724 cm−1 was pronounced both in the FTIR spectra and in the FT Raman spectra [44]. The intense Raman bands in the region of 1470–1200 cm−1 originated from CH2 deformational vibrations [45]. These bands are less pronounced in the FTIR spectra. These are the scissor vibration band at 1441 cm−1 (∼1457 cm−1 in infrared), or the wagging vibrations of (CH2)n near 1304 cm−1 (absent in infrared), the scissor vibrations of the —CH2–O— methylene groups at 1470 cm−1 (1469 cm−1 in infrared) and the—CH2–CO—methylene groups at 1418 cm−1 (1417 cm−1 in infrared). Similarly, the band at ∼ 1107–1108 cm−1 assigned to the C–C stretching vibration of alkane residues in PCL was more intense in FT-Raman. By contrast, the bands at 1238 and at 1165 cm−1, assigned to asymmetric and symmetric stretching vibrations of —CO–O–CH2— in the backbone, respectively [44], were more intense in FTIR than in FT Raman.
The vibrational bands of alaptide (e) and L-arginine (d) are insignificant in the FTIR and FT Raman spectra of the modified PCL membranes (b, c) (see figure 7). The amount of doped drug (1.5 %) adsorbed on the surface of the membrane is small, and the spectral features of peptide or amino acid therefore probably overlapped with the features of the nanofibrous matrix. However, several new bands were observed in the spectra of modified PCL membranes that confirm the presence of alaptide and/or L-arginine in the materials. There is a weak infrared band at 1682 cm−1 assigned to C = O vibration in L-arginine or alaptide and a weak Raman band at 1505 cm−1 assigned to > NH bending vibration of alaptide.
3.2. In vitro tests of nanofibrous layers
3.2.1. Membranes with alaptide
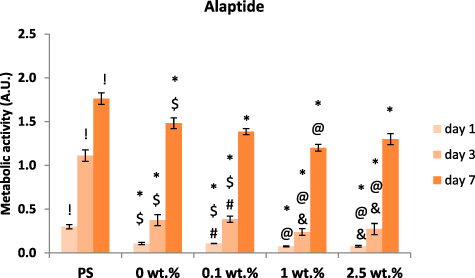
The results of the MTS assay indicate that the alaptide-containing nanofibrous membranes are colonized by NHDF cells in a similar manner as the control pure PCL membranes (figure 8). There is a decrease in metabolic activity on the samples with 1 wt.% and 2.5 wt.% of alaptide in comparison with the samples with 0.1 wt.% of alaptide and the pure PCL (0 wt.%) membrane. On the first and third days, the decrease was statistically significant for both 1 wt.% and 2.5 wt.% concentrations. On the seventh day, the metabolic activity of the cells on all tested membranes almost leveled; however, on the sample with 1 wt.% alaptide concentration, the difference was still significantly lower than for pure PCL. The comparison of all PCL membranes with the control tissue culture polystyrene (PS) shows slower colonization of the PCL membranes. Nevertheless, the PCL membrane proved to be a biocompatible substrate for cell cultivation. The results of the MTS assay were further confirmed by fluorescence microscopy images (figure 9). After the first day of cultivation, sparsely adhering NHDF cells can be recognized on the membranes, particularly on the membranes with alaptide. During the 3 d interval, the dividing cells slowly formed a reticular structure on the membranes, and after 7 d all the samples were completely covered by a confluent layer of cells.
Figure 8. Metabolic activity of NHDF cells in polystyrene (PS) wells and on nanofibrous PCL membranes with various concentrations of alaptide. The cells were cultivated for 1, 3 and 7 d. The concentration of alaptide is given in wt.%; 0 wt.% = pure PCL membrane (control). Depicted values are mean ± S.D. from 3 experiments with 3 samples each measured 3 times for each experimental group and time interval. Samples were compared by ANOVA with Tukey post-hoc test. Statistically significant results (alpha level = 5%) within the same cultivation interval are marked above the columns (! vs. all tested samples with alaptide; * vs. PS; @ vs. 0 wt. %; & vs. 0.1 wt. %; $ vs. 1 wt. %; # vs. 2.5 wt. %). All the statistically significant differences are valid inside the given cultivation interval (1, 3 or 7 d).
Download figure:
Standard image High-resolution imageFigure 9. NHDF cells cultivated for 1, 3 and 7 d on nanofibrous PCL membranes with various concentrations of alaptide.
Download figure:
Standard image High-resolution image3.2.2. Membranes with L-arginine
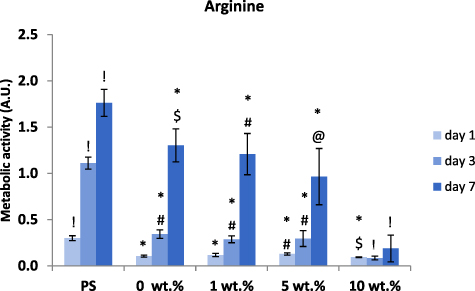
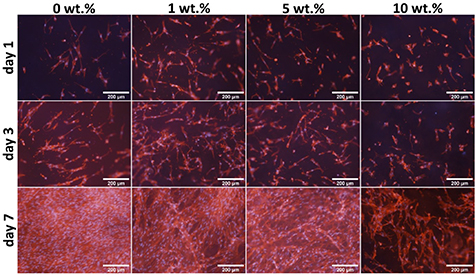
Using the MTS assay, we were able to find the toxic dose of L-arginine in the nanofibrous PCL samples (figure 10). The production process allowed us to incorporate a four times higher dose of L-arginine than of alaptide in the membranes, and the highest concentration, i.e. 10 wt.%, therefore proved to be significantly toxic for the NHDF cells. Nevertheless, there were still some cells adhered, proliferating and forming a reticular structure on the samples with 10 wt.% of L-arginine after 7 d of cultivation (see figure 11). A decrease in cell metabolic activity was also observable with lower concentrations (1 wt.% and 5 wt.%). We were able to detect a statistically significant difference in cell metabolic activity between the pure PCL (0 wt.%) membrane and the membrane with 5 wt.% of L-arginine. However, the decrease was not pronounced enough to consider the 5% concentration to be toxic. In addition, as indicated by the fluorescence images (figure 10), the cells on membranes with 5 wt.% L-arginine concentration were able to reach confluence on day 7 after seeding. The negative effect of the highest dose (10 wt.%) of L-arginine can also already be seen in the fluorescence images from the first day of cultivation. All the L-arginine-containing samples displayed some irregularities and discontinuities in the otherwise confluent layer of NHDF cells after 7 d of cultivation, in comparison with the cells on the pure PCL membrane.
Figure 10. Metabolic activity of NHDF cells in polystyrene (PS) wells and on nanofibrous PCL membranes with various concentrations of L-arginine. The cells were cultivated for 1, 3 and 7 d. The concentration of L-arginine is given in wt.%; 0% = pure PCL membrane (control). Depicted values are mean ± S.D. from 3 experiments with 3 samples each measured 3 times for each experimental group and time interval. Samples were compared by ANOVA with Tukey post-hoc test. Statistically significant results (alpha level = 5%) within the same cultivation period are marked above the columns (! vs. all other tested samples; * vs. polystyrene (PS); @ vs. 0 wt. %; & vs. 1 wt. %; $ vs. 5 wt. %; # vs. 10 wt. %). All the statistically significant differences are valid inside the given cultivation interval (1, 3 or 7 d).
Download figure:
Standard image High-resolution imageFigure 11. NHDF cells cultivated for 1, 3 and 7 d on the nanofibrous PCL membranes with various concentrations of L-arginine.
Download figure:
Standard image High-resolution image3.2.3. Membranes with alaptide and L-arginine
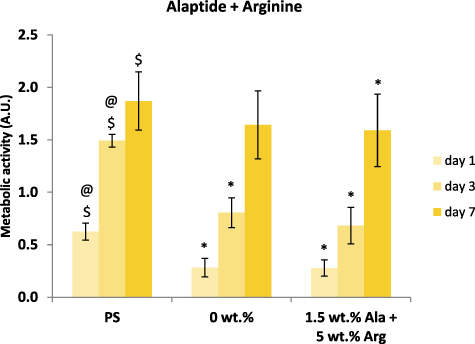
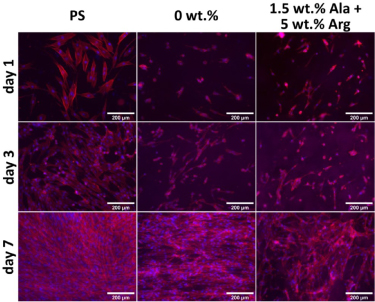
In order to study the combined effect of alaptide and L-arginine on NHDF cells, PCL membranes with non-toxic doses of both studied compounds were used (i.e. 1.5 wt.% of alaptide and 5 wt.% of L-arginine). Again, we measured the cell metabolic activity using the MTS test, and the results showed very little if any effect of the combined presence of alaptide and L-arginine, in comparison with the pure PCL membrane (figure 12). However, the cell metabolic activity on all PCL membranes was usually lower than the activity on the control cell culture polystyrene dishes. The fluorescence microscopy images (figure 13) showed a different morphology of the cell layer on the alaptide and L-arginine-containing membrane, where void spaces in the cell layer were apparent as in the experiments with only L-arginine in the PCL membranes. The NHDF cells on the pure PCL membrane appear to be oriented along the nanofibers of the membrane.
Figure 12. Metabolic activity of NHDF cells on nanofibrous PCL membranes with 1.5 wt.% of alaptide and 5 wt.% of L-arginine (A + R) in comparison with pure PCL nanofibrous membrane (PCL) and tissue culture polystyrene (PS). Depicted values are mean ± S.D. from 3 experiments with 3 samples each measured 3 times for each experimental group and time interval. Samples were compared by ANOVA with Tukey post-hoc test. Statistically significant results (alpha level = 5%) within the cultivation period of 1, 3 and 7 d are marked above the columns (* vs. PS; @ vs. 0 wt.% pure PCL membrane; $ vs. the corresponding samples with alaptide and L-arginine).
Download figure:
Standard image High-resolution imageFigure 13. NHDF cells cultivated on control polystyrene (PS), on pure PCL membranes (0 wt.%) and on a PCL membrane with 1.5 wt.% of alaptide and 5 wt.% of L-arginine (1.5 wt.% Ala + 5 wt.% Arg).
Download figure:
Standard image High-resolution image3.3. In vivo preclinical tests on rats
The in vitro tests of alaptide did not prove any significant cytotoxicity for the selected concentrations (0.1–2.5 wt.%), and we also did not observe any significant differences in cell growth. However, 10 wt.% of L-arginine already appeared to be cytotoxic. For the in vivo experiments, therefore, membranes containing only 1.5 wt.% of alaptide or a mixture of 1.5 wt.% of alaptide and 5 wt.% of L-arginine were prepared. Visual monitoring of the in vivo wound healing process for the control and experimental groups is presented in figures 14–16. These images were chosen as typical cases for visualizing each group consisting of three white lab rats isolated in individual cages under the same conditions of maintenance and feeding. We tested two types of wounds. The first type was the healing of burn damage, and the second type was the healing of deep cut wounds.
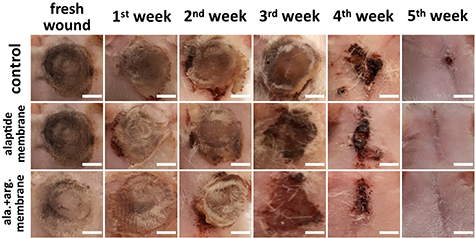
Figure 14. Representative images of the healing process of burn wounds without treatment (control), and treated with a PCL membrane modified with alaptide (1.5 wt.%; alaptide membrane) or modified with an alaptide/L-arginine mixture (1.5 wt.%/5 wt.%; ala. + arg. membrane). Scale bar = 10 mm.
Download figure:
Standard image High-resolution imageFigure 15. Time dependence of the wound area during the healing of skin burns and skin deep incisions without treatment (untreated), and treated with PCL membranes modified either with alaptide (1.5 wt.%; PCL/alaptide) or with the alaptide/L-arginine mixture (1.5 wt.%/5 wt.%; alaptide/Arg). Depicted values are mean ± S.D. from three animals for each experimental group and time interval. The statistical significance was calculated for each day separately using ANOVA with Tukey's post hoc test, α level = 5%, #—significant difference between the untreated sample and PCL/alaptide/Arg, *—significant difference between the untreated sample and both PCL/alaptide and PCL/alaptide/Arg.
Download figure:
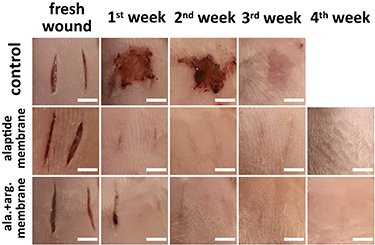
Standard image High-resolution imageFigure 16. Representative images of the healing process of deep incisions without treatment (control), and treated with a PCL membrane modified with alaptide (1.5 wt.%; alaptide membrane) or with an alaptide/L-arginine mixture (1.5 wt.%/5 wt.%; ala. + arg. membrane). Scale bar = 10 mm.
Download figure:
Standard image High-resolution image3.3.1. Skin burns
The process of healing burns was monitored visually for 7, 14, 21, 28 and 35 d, in order to estimate the effect of the modified PCL nanofibers on burn recovery figure 14. In all cases, a burn injury led to marked skin necrosis and to the appearance of erythema. Immediately after the injury, the burns were untreated or were covered with PCL nanofibers containing the additives (second column on the left). On the seventh day after the burn injury, the eschar was noted in the wounds. For the treated groups, the eschar was densely covered with gradually degrading PCL. The gradual destruction of the material led to the release of the additives, which were then absorbed by the wound tissues, which stimulated the healing process. Over the following days, the use of PCL doped with alaptide or alaptide/L-arginine significantly improved the wound closure and re-epithelialization during the burn wound healing process. On the 35th day after the burn, almost complete wound healing was achieved using the modified PCL membranes. In this case, the healing was significantly faster than for the untreated group, which did not achieve complete closure of the wound. The dependence of the wound area on the time after skin burn injury (figure 15) illustrated how the healing process proceeded in all experimental groups. It was evident that, in contrast to the untreated group, the use of PCL doped with the alaptide/L-arginine combination did not lead to any significant increase in the wound area during the first week after injury. In this case, the wound area was significantly smaller than the area of untreated wounds. After 4 weeks (day 28), the size of the wounds treated with PCL membranes with alaptide/L-arginine dropped almost to zero and was significantly smaller than in the untreated wounds, while in membranes loaded only with alaptide, similar low values were obtained later (after 5–6 weeks). Therefore, the use of PCL membranes with alaptide/L-arginine mixture was more effective than the membranes loaded with pure alaptide for healing skin burns, and the two biomolecules showed a synergetic effect.
3.3.2. Skin incisions
In the control group, the healing of ∼ 4 mm deep incisions passed without any treatment. The dependence of the size of the wound area on the time after skin incision (figure 15) demonstrated a significant increase in the eschar area in this group during the first week. 7 d after the incision, the state of the wounds corresponded to the proliferative phase of healing. This stage is characterized by the appearance of a red granulation tissue with an uneven texture, consisting of collagen and other extracellular matrix (ECM) molecules. This tissue forms a carrier platform for the formation of new connective tissue and skin [46–48]. In the proliferative phase, the wound shrinks while new tissues are formed. Dark granulation tissue may be a sign of infection, ischemia, or poor perfusion. From our experiments, it is evident that this process continues for at least 14 d. At the end of the proliferative stage, epithelial cells reappear on the surface of the wound. In the next phase, the collagen is converted from type III to type I and the wound closes [46]. During this epithelization phase, the collagen fibers are cross-linked, which reduces the scar thickness and makes the skin area stronger. In the case of the control non-treated group in our study, remodeling was observed 21 d after the injury.
In the treated groups, the process of wound healing was observed after the use of PCL membranes modified with alaptide (1.5 wt.%) or with an alaptide (1.5 wt.%)/L-arginine (5 wt.%) mixture (figures 15, 16). In both cases, evident acceleration of the healing process was observed. At further stages in all the groups, the healing process led to a gradual decrease in the wound areas, and the decrease was significantly more pronounced for the animals treated with the modified PCL membranes. The epithelialization phase of the treated wounds was already observed 7 d after injury. In addition to the healing effects of alaptide and arginine, it is likely that wound treatment with PCL nanotextile may accelerate the healing process by maintaining moisture, cleanliness and protection of the surface of the wound from repeated damage and infection.
4. Discussion
In this study, we have examined the in vitro cell response and the in vivo healing abilities of alaptide- and L-arginine-incorporated nanofibrous PCL membranes. Nanofibrous membranes proved to be optimal covers for wound healing, due to their structural (architectural) similarity to the native skin ECM [49]. PCL is already a well-established biocompatible, biodegradable and electrospinnable polymer [50, 51]. PCL nanofibrous membranes are advantageous as wound dressings because of their ability to extract the exudate from the wound and because of their gas (oxygen) permeability; however, adding some healing-promoting compounds to the wound dressing that is in direct contact with the healing tissue can accelerate the healing process and prevent wound infections.
Two additives have been investigated in this work. Alaptide—a partially forgotten compound—was chosen mainly due to its positive influence on epidermal regeneration and due to its low acute toxicity [14]. This dipeptide is already used in veterinary practice as a component of a skin healing ointment. L-arginine was also chosen because of its positive effect on wound healing. As a donor of nitric oxide, L-arginine plays a pivotal role in skin repair [18]. All nanofibrous membranes were successfully fabricated with two types of dipeptide and amino acid additives incorporated into the fibers. Due to the fact that both alaptide and L-arginine are not fully soluble in the solvent system used for PCL electrospinning, the electrospinning process took place mainly from a suspension. Both additives are therefore present in the PCL fibers at least partly in the form of micro-particles trapped by polymeric fibers. Only 0.1 wt.% of alaptide was completely dissolved. The membranes with 1 and 2.5 wt.% alaptide released around 60% of the alaptide within the first hour under in vitro conditions in a so-called 'burst release'. A burst release was also observed in the membrane with 0.1 wt.% of alaptide; however, only up to 40% of the alaptide was released, and this was followed by long-term slow release of the drug. A burst release could also be observed for L-arginine in the plots of the change in pH in demineralized water. This sudden change in concentration and also in pH for L-arginine can have a negative influence on the cells under in vitro conditions. Slower controlled drug release would therefore be more beneficial. This type of membrane behavior is can be achieved by electrospinning from a solution rather than from a suspension [29, 31]. The suspension of L-arginine tends to be very unstable and we observed the formation of L-arginine agglomerates. This phenomenon affected the homogeneity of the distribution of the drug in the PCL membrane.
In general, the results from the in vitro experiments did not indicate any improvement in the proliferation of the dermal fibroblast on the PCL membranes with incorporated alaptide or L-arginine. With a rising concentration of alaptide, we even observed a slight decrease in the cell metabolic activity. This decrease was statistically significant for the PCL membrane with 1 wt.% of alaptide in comparison with the pure PCL membrane. However, no significant difference in cell metabolic activity was found between the pure PCL membrane and the 2.5 wt.% sample. At the same time, the cell metabolic activity on the samples with 2.5 wt. % of alaptide was higher than on the samples with 1 wt.%. We observed this tendency in all repeated experiments. This observation could in turn indicate that concentrations higher than 2.5 wt.% can support the healing process. This concentration can be increased, but most of the drug will, in any case, be released within the first two hours. Taken together, our findings are in accordance with the results of a recent study, in which alaptide was investigated as a potential chemical permeation enhancer for transdermal drug delivery. A LIVE/DEAD test based on Calcein AM (i.e. a substrate for esterase enzymes in living cells), performed in vitro on human foreskin fibroblasts and on two cancer cell lines, indicated no significant cytotoxicity of alaptide [52].
The metabolic activity of fibroblasts cultivated on PCL membranes with L-arginine clearly decreased with increasing L-arginine concentration. The cell metabolic activity on the membrane with 10 wt.% of L-arginine and the fluorescence images clearly indicate that this membrane is toxic for the cells. A possible explanation may be a high local concentration of L-arginine. Although this amino acid contains a basic side chain, its acidic character prevailed, and its release from PCL nanofibrous membranes reduced the pH of the demineralized water. In our study, L-arginine was used in the form of L-arginine monohydrochloride. In water-based environments, this compound is converted into citrulline and nitric oxide, which is accompanied with formation of hydrochloric acid (HCl see figure 1). Thus, L-arginine monohydrochloride can produce local acidosis (particularly in a static culture system with a relatively small amount of cell culture media), which could have a negative effect on cell viability and growth. It is therefore better to find a more suitable solvent system to obtain a long-term drug release profile. High burst release can be advantageous in the case of a bacterial infection where a higher concentration of a selected drug is needed for a short period of time, and only a small amount of the drug is sufficient to protect the wound against any further infection [8]. Another possible explanation for the cytotoxicity of L-arginine is the dual effect of nitric oxide generated from this molecule. On the one hand, nitric oxide mediates wound healing by supporting epithelialization, angiogenesis and collagen deposition, but on the other hand, this compound can cause oxidative damage and death of cells (for a review, see [53]). In addition, dicationic arginine-diglyceride surfactants, developed for use in pharmaceutical and cosmetic preparations, showed cytotoxicity for murine 3T3 fibroblasts and human HaCaT keratinocytes in vitro, although this cytotoxicity was much lower than the cytotoxicity of commercially available surfactants [54].
In contrast to the neutral or somewhat negative effects of alaptide and L-arginine on the growth of human dermal fibroblasts in vitro, both compounds markedly improved the healing of experimental wounds in vivo in rats. As follows from figure 15, the healing of the untreated burn wounds lasted 42 d, i.e. 6 weeks, where the size of the wound area was close to zero. In wounds treated with membranes loaded with a combination of alaptide and L-arginine, this value became close to zero after 28 d, i.e. after 4 weeks. Thus, the membranes with the combination of alaptide and L-arginine, markedly accelerated the wound healing. In the incision wounds, the size of the wound area became close to zero after 28 d (4 weeks) in both treated and untreated wounds. However, in the untreated wounds, a relatively large eschar was formed and persisted for almost the whole period of healing. However, the size of the wounds treated with PCL membranes with alaptide or with the alaptide/arginine combination decreased rapidly immediately after the wound was created and almost without eschar formation, as demonstrated in figure 16. This is in accordance with many experimental studies, in which alaptide stimulated epithelialization and epidermal regeneration (for a review, see [11, 14]. Alaptide is an analogue of the melanocyte-stimulating hormone release-inhibiting factor (MIF), also referred to as macrophage migration inhibitory factor (MIF), which is known to be a key player in cutaneous biology and wound healing. MIF is a key effector of the beneficial effects of estrogen on wound repair [55], and it induces the proliferation of keratinocytes in vitro and in vivo [56].
Arginine is also known to have positive effects on wound healing in vivo, e.g. after direct local administration of an arginine solution into excisional wounds in rats [57], or after administration through gels containing lignin nanofibers [27] or bacterial cellulose nanofibers [28], into full-thickness skin wounds in rats. It should be pointed out that L-arginine incorporated into both nanofibrous gels accelerated wound healing better than L-arginine in a solution. This was attributed to the moist environment provided by nanofibrous gels, and their structural similarity to ECM [27, 28]. The main mechanism of the wound healing effect of L-arginine is considered to be the generation of nitric oxide from this molecule by constitutive and inducible nitric oxide synthases (cNOS and iNOS, respectively), produced by cells. However, arginine is also a substrate of arginase, which converts it into ornithine, another important molecule in wound healing. Ornithine is a substrate for the synthesis of polyamines, which are important in cell proliferation. Proline, derived from ornithine, is a key molecule for collagen synthesis (for a review, see [53]). Arginine-coated nanofibers can also support the viability and growth of cells in vitro. L-arginine conjugated with polyurethane nanofibers stimulated proliferation and prevented H2O2-induced death in skeletal muscle satellite cells co-cultured with fibroblasts [26].
5. Conclusion and further perspectives
In this study, we used spirocyclic dipeptide alaptide and single amino-acid L-arginine as therapeutic additives in PCL electrospun nanofibrous membranes intended as wound dressings. The membranes were characterized for their physical and chemical properties, and then they were tested in vitro on human dermal fibroblasts and in vivo on rats. PCL is one of the most promising polymers for use as a wound dressing due to its tunable biodegradability and cytocompatibility. We did not observe any statistically significant increase in the diameters of the fibers with increasing drug concentration. Some defects found within the nanofibrous structure had similar shapes and sizes as L-arginine and alaptide particles. This is mainly due to electrospinning from suspensions (with the exception of 0.1 wt.% alaptide). Concentrations of both additives selected by in vitro testing were then tested on rats for two types of wounds, i.e. burn and incision wounds. Alaptide did not prove to have any cytotoxicity for all selected concentrations in vitro. L-arginine proved to be cytotoxic for concentrations higher than 5 wt.% in solution. Alaptide seemed to be a more promising molecule; however, it is necessary to take into account that the concentrations of alaptide tested in the membrane were lower than for L-arginine. L-arginine seems to agglomerate and change the pH by dissolving into the cultivation medium, which has a negative effect on its biological activity. However, we have observed improved wound closure and re-epithelization of burn injuries after the use of PCL nanofibrous membranes modified with alaptide or with a combination of alaptide and arginine. A similar healing effect was also observed for incision wounds. We believe that the main potential for the use of modified nanofibrous membranes is in the treatment of large wounds, where supportive material is needed. In real situations, these wounds are often infected and they can cause sepsis, mainly in the case of burn injuries. Modifying nanofibers not only with wound healing drugs but also with antibacterial drugs is therefore crucial for clinical applications of the nanofibrous membranes.
Acknowledgments
The authors would like to express their gratitude for financial support provided by the Czech Science Foundation (GAČR, Project No. 17-02448S 'Improved growth of human skin cells on biomimetic nanofibrous matrices for active wound healing)'. Mr Robin Healey is gratefully acknowledged for his language revision of the manuscript. We also thank Dr Pavla Poučková and Dr Marie Zadinová for help with the in vivo tests.
Author disclosure statement
The authors have no conflicts of interest to declare.