Abstract
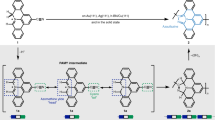
Antiaromatic polycyclic conjugated hydrocarbons (PCHs) are attractive research targets because of their interesting structural, electronic and magnetic properties. Unlike aromatic compounds, the synthesis of antiaromatic PCHs is challenging because of their high reactivity and lack of stability, which stems from the small energy gap between their highest occupied and lowest unoccupied molecular orbitals. Here we describe a strategy for the introduction of antiaromatic units in PCHs via thermally selective intra- and intermolecular ring-rearrangement reactions of dibromomethylene-functionalized molecular precursors upon sublimation on a hot Au(111) metal surface, not available in solution chemistry. The synthetic value of these reactions is proven by the integration of pentalene segments into acene-based precursors, which undergo intramolecular ring rearrangement, and the formation of π-conjugated ladder polymers, linked through cyclobutadiene connections, due to ring-rearrangement and homocoupling reactions of indenofluorene-based precursors. The reaction products are investigated by scanning tunnelling microscopy and non-contact atomic force microscopy, and mechanistic insights are unveiled by computational studies.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during this study are available at the IMDEA Nanociencia repository (https://repositorio.imdeananociencia.org).
Code availability
The Fireball software package is available at https://github.com/fireball-QMD and the PP-SPM software package can be downloaded at https://github.com/Probe-Particle/ppafm#probe-particle-model.
References
Breslow, R., Brown, J. & Gajewski, J. J. Antiaromaticity of cyclopropenyl anions. J. Am. Chem. Soc. 89, 4383–4390 (1967).
Breslow, R. Antiaromaticity. Acc. Chem. Res. 6, 393–398 (1973).
Randic, M. Aromaticity and conjugation. J. Am. Chem. Soc. 99, 444–450 (1977).
von E. Doering, W. & Detert, F. L. Cycloheptatrienylium oxide. J. Am. Chem. Soc. 73, 876–877 (1951).
Zeng, Z. et al. Pro-aromatic and anti-aromatic π-conjugated molecules: an irresistible wish to be diradicals. Chem. Soc. Rev. 44, 6578–6596 (2015).
Cao, J. et al. The impact of antiaromatic subunits in [4n + 2] π-systems: bispentalenes with [4n + 2] π-electron perimeters and antiaromatic character. J. Am. Chem. Soc. 137, 7178–7188 (2015).
Kawase, T. et al. Dinaphthopentalenes: pentalene derivatives for organic thin-film transistors. Angew. Chem. 122, 7894–7898 (2010).
Chase, D. T. et al. 6,12-Diarylindeno[1,2-b]fluorenes: syntheses, photophysics and ambipolar OFETs. J. Am. Chem. Soc. 134, 10349–10352 (2012).
Dai, G., Chang, J., Jing, L. & Chi, C. Diacenopentalene dicarboximides as new n-type organic semiconductors for field-effect transistors. J. Mater. Chem. C 4, 8758–8764 (2016).
Grenz, D. C., Schmidt, M., Kratzert, D. & Esser, B. Dibenzo[a,e]pentalenes with low-lying LUMO energy levels as potential n-type materials. J. Org. Chem. 83, 656–663 (2018).
Shin, J.-Y., Yamada, T., Yoshikawa, H., Awaga, K. & Shinokubo, H. An antiaromatic electrode-active material enabling high capacity and stable performance of rechargeable batteries. Angew. Chem. Int. Ed. 53, 3096–3101 (2014).
Mei, J., Diao, Y., Appleton, A. L., Fang, L. & Bao, Z. Integrated materials design of organic semiconductors for field-effect transistors. J. Am. Chem. Soc. 135, 6724–6746 (2013).
Dai, G. et al. Dianthraceno[a,e]pentalenes: synthesis, crystallographic structures and applications in organic field-effect transistors. Chem. Commun. 51, 503–506 (2015).
Liu, C. et al. Diaceno[a,e]pentalenes: an excellent molecular platform for high-performance organic semiconductors. Chem. Eur. J. 21, 17016–17022 (2015).
Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Shen, Q., Gao, H.-Y. & Fuchs, H. Frontiers of on-surface synthesis: from principles to applications. Nano Today 13, 77–96 (2017).
Urgel, J. I. et al. On-surface synthesis of heptacene organometallic complexes. J. Am. Chem. Soc. 139, 11658–11661 (2017).
Zugermeier, M. et al. On-surface synthesis of heptacene and its interaction with a metal surface. Nanoscale 9, 12461–12469 (2017).
Zuzak, R. et al. Higher acenes by on-surface dehydrogenation: from heptacene to undecacene. Angew. Chem. Int. Ed. 57, 10500–10505 (2018).
Urgel, J. I. et al. On-surface light-induced generation of higher acenes and elucidation of their open-shell character. Nat. Commun. 10, 861 (2019).
Krüger, J. et al. Decacene: on-surface generation. Angew. Chem. Int. Ed. 56, 11945–11948 (2017).
Eisenhut, F. et al. Dodecacene generated on surface: reopening of the energy gap. ACS Nano 14, 1011–1017 (2020).
Eimre, K. et al. On-surface synthesis and characterization of nitrogen-substituted undecacenes. Nat. Commun. 13, 511 (2022).
Mishra, S. et al. Tailoring bond topologies in open-shell graphene nanostructures. ACS Nano 12, 11917–11927 (2018).
Rogers, C. et al. Closing the nanographene gap: surface-assisted synthesis of peripentacene from 6,6′-bipentacene precursors. Angew. Chem. Int. Ed. 54, 15143–15146 (2015).
Sánchez-Grande, A. et al. Unravelling the open-shell character of peripentacene on Au(111). J. Phys. Chem. Lett. 12, 330–336 (2021).
Biswas, K. et al. Synthesis and characterization of peri-heptacene on a metallic surface. Angew. Chem. Int. Ed. 61, e202114983 (2022).
Majzik, Z. et al. Studying an antiaromatic polycyclic hydrocarbon adsorbed on different surfaces. Nat. Commun. 9, 1198 (2018).
Di Giovannantonio, M. & Fasel, R. On-surface synthesis and atomic scale characterization of unprotected indenofluorene polymers. J. Polym. Sci. 60, 1814–1826 (2022).
Su, J., Telychko, M., Song, S. & Lu, J. Triangulenes: from precursor design to on-surface synthesis and characterization. Angew. Chem. Int. Ed. 59, 7658–7668 (2020).
Dong, L., Liu, P. N. & Lin, N. Surface-activated coupling reactions confined on a surface. Acc. Chem. Res. 48, 2765–2774 (2015).
Kinikar, A. et al. On-surface polyarylene synthesis by cycloaromatization of isopropyl substituents. Nat. Synth. 1, 289–296 (2022).
Biswas, K. et al. Interplay between π-conjugation and exchange magnetism in one-dimensional porphyrinoid polymers. J. Am. Chem. Soc. 144, 12725–12731 (2022).
Shiotari, A. et al. Strain-induced skeletal rearrangement of a polycyclic aromatic hydrocarbon on a copper surface. Nat. Commun. 8, 16089 (2017).
Lohr, T. G. et al. On-surface synthesis of non-benzenoid nanographenes by oxidative ring-closure and ring-rearrangement reactions. J. Am. Chem. Soc. 142, 13565–13572 (2020).
Mallada, B. et al. On-surface strain-driven synthesis of nonalternant non-benzenoid aromatic compounds containing four- to eight-membered rings. J. Am. Chem. Soc. 143, 14694–14702 (2021).
Stetsovych, O. et al. From helical to planar chirality by on-surface chemistry. Nat. Chem. 9, 213–218 (2017).
Albrecht, F. et al. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science 377, 298–301 (2022).
Cirera, B. et al. Thermal selectivity of intermolecular versus intramolecular reactions on surfaces. Nat. Commun. 7, 11002 (2016).
Tang, Y. et al. On-surface debromination of 2,3-bis(dibromomethyl)- and 2,3-bis(bromomethyl)naphthalene: dimerization or polymerization? Angew. Chem. Int. Ed. 61, e202204123 (2022).
Kawai, S. et al. Competing annulene and radialene structures in a single anti-aromatic molecule studied by high-resolution atomic force microscopy. ACS Nano 11, 8122–8130 (2017).
Kawai, S. et al. An endergonic synthesis of single Sondheimer-Wong diyne by local probe chemistry. Angew. Chem. Int. Ed. 59, 10842–10847 (2020).
Li, D.-Y. et al. Ladder phenylenes synthesized on Au(111) surface via selective [2 + 2] cycloaddition. J. Am. Chem. Soc. 143, 12955–12960 (2021).
Jacobse, P. H. et al. Pseudo-atomic orbital behavior in graphene nanoribbons with four-membered rings. Sci. Adv. 7, eabl5892 (2021).
Di Giovannantonio, M. et al. On-surface synthesis of antiaromatic and open-shell indeno[2,1-b]fluorene polymers and their lateral fusion into porous ribbons. J. Am. Chem. Soc. 141, 12346–12354 (2019).
de la Torre, B. et al. Tailoring π-conjugation and vibrational modes to steer on-surface synthesis of pentalene-bridged ladder polymers. Nat. Commun. 11, 4567 (2020).
Liu, M. et al. Graphene-like nanoribbons periodically embedded with four- and eight-membered rings. Nat. Commun. 8, 14924 (2017).
Sánchez‐Sánchez, C. et al. On-surface synthesis and characterization of acene-based nanoribbons incorporating four-membered rings. Chem. Eur. J. 25, 12074–12082 (2019).
Fan, Q. et al. Nanoribbons with nonalternant topology from fusion of polyazulene: carbon allotropes beyond graphene. J. Am. Chem. Soc. 141, 17713–17720 (2019).
Sánchez-Grande, A. et al. On-surface synthesis of ethynylene-bridged anthracene polymers. Angew. Chem. 131, 6631–6635 (2019).
Cirera, B. et al. Tailoring topological order and π-conjugation to engineer quasi-metallic polymers. Nat. Nanotechnol. 15, 437–443 (2020).
Sánchez‐Grande, A. et al. Diradical organic one‐dimensional polymers synthesized on a metallic surface. Angew. Chem. Int. Ed. 59, 17594–17599 (2020).
Biswas, K. et al. On-surface synthesis of doubly-linked one-dimensional pentacene ladder polymers. Chem. Commun. 56, 15309–15312 (2020).
Martín-Fuentes, C. et al. Cumulene-like bridged indeno[1,2-b]fluorene π-conjugated polymers synthesized on metal surfaces. Chem. Commun. 57, 7545–7548 (2021).
Sánchez-Grande, A. et al. Surface-assisted synthesis of N-containing π-conjugated polymers. Adv. Sci. 9, 2200407 (2022).
Urgel, J. I. et al. On‐surface synthesis of cumulene‐containing polymers via two‐step dehalogenative homocoupling of dibromomethylene‐functionalized tribenzoazulene. Angew. Chem. Int. Ed. 59, 13281–13287 (2020).
Di Giovannantonio, M. et al. On-surface growth dynamics of graphene nanoribbons: the role of halogen functionalization. ACS Nano 12, 74–81 (2018).
Di Giovannantonio, M. et al. On-surface synthesis of indenofluorene polymers by oxidative five-membered ring formation. J. Am. Chem. Soc. 140, 3532–3536 (2018).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Zhang, H. et al. On-surface synthesis of rylene-type graphene nanoribbons. J. Am. Chem. Soc. 137, 4022–4025 (2015).
Zhang, H. et al. Surface supported gold–organic hybrids: on-surface synthesis and surface directed orientation. Small 10, 1361–1368 (2014).
Levi, Z. U. & Tilley, T. D. Synthesis and electronic properties of extended, fused-ring aromatic systems containing multiple pentalene units. J. Am. Chem. Soc. 132, 11012–11014 (2010).
Sekine, K. et al. Gold-catalyzed facile synthesis and crystal structures of benzene-/naphthalene-based bispentalenes as organic semiconductors. Chem. Eur. J. 25, 216–220 (2019).
Tavakkolifard, S. et al. Gold-catalyzed regiospecific annulation of unsymmetrically substituted 1,5-diynes for the precise synthesis of bispentalenes. Chem. Eur. J. 25, 12180–12186 (2019).
Frederickson, C. K., Zakharov, L. N. & Haley, M. M. Modulating paratropicity strength in diareno-fused antiaromatics. J. Am. Chem. Soc. 138, 16827–16838 (2016).
Wilbuer, J., Grenz, D. C., Schnakenburg, G. & Esser, B. Donor- and acceptor-functionalized dibenzo[a, e]pentalenes: modulation of the electronic band gap. Org. Chem. Front. 4, 658–663 (2017).
Pavliček, N. et al. Polyyne formation via skeletal rearrangement induced by atomic manipulation. Nat. Chem. 10, 853–858 (2018).
Giessibl, F. J. Atomic resolution on Si(111)-(7 × 7) by noncontact atomic force microscopy with a force sensor based on a quartz tuning fork. Appl. Phys. Lett. 76, 1470–1472 (2000).
Bartels, L. et al. Dynamics of electron-induced manipulation of individual CO molecules on Cu(111). Phys. Rev. Lett. 80, 2004–2007 (1998).
Horcas, I. et al. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007).
Pearlman, D. A. et al. AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput. Phys. Commun. 91, 1–41 (1995).
Case, D. A. et al. The AMBER biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Lewis, J. P. et al. Advances and applications in the FIREBALL ab initio tight-binding molecular-dynamics formalism. Phys. Status Solidi B 248, 1989–2007 (2011).
Mendieta-Moreno, J. I. et al. Fireball/AMBER: an efficient local-orbital DFT QM/MM method for biomolecular systems. J. Chem. Theory Comput. 10, 2185–2193 (2014).
Darve, E. & Pohorille, A. Calculating free energies using average force. J. Chem. Phys. 115, 9169–9183 (2001).
Izrailev, S. et al. in Computational Molecular Dynamics: Challenges, Methods, Ideas (eds Deuflhard, P. et al.) 39–65 (Springer, 1999).
Pastor, R. W., Brooks, B. R. & Szabo, A. An analysis of the accuracy of Langevin and molecular dynamics algorithms. Mol. Phys. 65, 1409–1419 (1988).
WHAM (Grossfield Laboratory); http://membrane.urmc.rochester.edu/?page_id=126
Kumar, S., Rosenberg, J. M., Bouzida, D., Swendsen, R. H. & Kollman, P. A. THE weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 13, 1011–1021 (1992).
Krejčí, O., Hapala, P., Ondráček, M. & Jelínek, P. Principles and simulations of high-resolution STM imaging with a flexible tip apex. Phys. Rev. B 95, 045407 (2017).
Herges, R. & Geuenich, D. Delocalization of electrons in molecules†. J. Phys. Chem. A 105, 3214–3220 (2001).
Chen, Z., Wannere, C. S., Corminboeuf, C., Puchta, R. & von R. Schleyer, P. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105, 3842–3888 (2005).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, 2016).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Acknowledgements
This project has received funding from Comunidad de Madrid (projects QUIMTRONIC-CM (Y2018/NMT-4783) and NanoMagCost (P2018/NMT-4321)), an ERC Consolidator Grant (ELECNANO, 766555), ERC (SyG TOMATTO ERC-2020-951224) and Ministerio de Ciencia, Innovacion y Universidades (projects SpOrQuMat (PGC2018-098613-B-C21), CTQ2017-83531-R, PID2019-108532GB-I00, PID2020-114653RB-I00 and CTQ2016-81911-REDT). We acknowledge the support from the ‘(MAD2D-CM)-UCM’ and the ‘(MAD2D-CM)-IMDEA-Nanociencia’ projects funded by Comunidad de Madrid, by the Recovery, Transformation and Resilience Plan, and by NextGenerationEU from the European Union. IMDEA Nanociencia is appreciative of support from the ‘Severo Ochoa’ Programme for Centers of Excellence in R&D (MINECO, grant nos. SEV-2016-0686 and CEX2020-001039-S). Q.C., D.S.-P. and P.J. acknowledge funding support from the CzechNanoLab Research Infrastructure supported by MEYS CR (LM2023051) and GACR project no. 20-13692X. Computational resources were provided by the e-INFRA CZ project (ID 90140), supported by the Ministry of Education, Youth and Sports of the Czech Republic. A.S.-G. acknowledges funding from the ‘Ministerio de Universidades’ for the ‘Plan de Recuperación, Transformación y Resiliencia’ under Margarita Salas grant agreement CA1/RSUE/2021-00369. J.I.U. acknowledges the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement no. 886314. We acknowledge B. Cirera for fruitful discussions.
Author information
Authors and Affiliations
Contributions
E.P.-E., A.B., P.J., J.I.U., N.M. and D.E. conceived the project and designed the experiments. D.J.V. and J.S. synthesized the precursors. E.P.-E., A.B., A.S.-G. and B.d.l.T. carried out the experiments. Q.C., D.S.-P., P.M. and J.I.M.-M. performed the theoretical calculations. The experimental data and theoretical results were analysed and discussed by all the authors. E.P.-E., A.B., P.J., J.I.U., N.M. and D.E. wrote the paper, with contributions from all the authors. D.E. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Discussion, Supplementary Figs. 1–17 and References.
Supplementary Video 1
Simulation of reaction mechanism for precursor 1.
Supplementary Data 1
Source data for SI.
Source data
Source Data Fig. 2
Unprocessed images for Fig. 2.
Source Data Fig. 3
Unprocessed images for Fig. 3.
Source Data Fig. 4
Unprocessed images for Fig. 4.
Source Data Fig. 6
Unprocessed images for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez-Elvira, E., Barragán, A., Chen, Q. et al. Generating antiaromaticity in polycyclic conjugated hydrocarbons by thermally selective skeletal rearrangements at interfaces. Nat. Synth 2, 1159–1170 (2023). https://doi.org/10.1038/s44160-023-00390-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00390-8