Abstract
Plants adapted to nutrient-poor temperate peatlands exhibit relatively low foliar N and P contents and resorb N and P from ageing leaves very efficiently to minimize their nutrient losses through litter fall. Changes in nutrient resorption efficiency due to an expectable increase of temperature may affect nutrient cycling in peatlands. We used an elevational gradient as a proxy for the effect of ongoing climatic change and compared the foliar N and P resorption efficiencies (REN, REP) of two co-occurring typical plant dominants (Molinia caerulea and Vaccinium uliginosum) at four microsites of each of two acidic peatbogs in southern Bohemia, Czechia, at two elevations differing by ca 500 m. No significant difference in soil nutrient content was found between the two sites. Foliar N and P contents in mature leaves in both species did not depend on the elevational gradient and were mostly not correlated with the inorganic soil nutrients. The REN (70–78%) and REP values (61–70%) in Vaccinium were markedly lower than those in Molinia (84–85% and 92–94%, respectively). In line with literature data, the peatland dominants Molinia and Vaccinium possess different strategies of foliar N and P resorption from ageing leaves. High REN and REP in Molinia obviously underlie its strong dominance in unmanaged peatland habitats. No elevational difference in both REN and REP was found in either species, indicating that the resorption efficiencies of these species are not expected to change markedly under the projected scenario of increasing temperature and lengthening growing season.
Similar content being viewed by others
Introduction
Nutrient resorption from senescing leaves and / or shoots enables plants to decrease the nutrient losses associated with biomass turnover and represents a significant component of their mineral nutrient economy (Güsewell 2004, 2005a,b). Plants growing in nutrient-poor habitats might minimize their relative nutrient losses in senescent leaves / shoots by increasing the fraction of nutrients reutilized in mature leaves, that is, the ‘resorption efficiency’ (RE), or decreasing the final nutrient content in senescent biomass, that is, the ‘resorption proficiency’ (Killingbeck 1996). Another strategy is to evolve a long life span of organs (Aerts et al. 1999). Plants adapted to growing in nutrient-poor peatlands (bogs and fens) resorb N and P very efficiently. Aerts et al. (1999) and Güsewell (2005b) state mean RE values for N (REN) of ca 40–55% and P (REP) ca 60–80% (cf. Aerts 1989; Aerts and Caluwe 1989) for dozens of peatland species. Carnivorous plants resorb N and P even more efficiently (Adamec 2002). Usually, REN closely positively correlates with REP (Killingbeck 1996; He et al. 2020). Plant species growing in nutrient-poor habitats do not always resorb N and P more efficiently than those in nutrient-rich habitats and their resorption efficiency may not correlate with soil nutrient content (Aerts 1996; Güsewell 2005b and the literature therein).
As temperature influences various plant processes differently (e.g. mineral nutrient uptake, photosynthesis or growth), it might also influence foliar N and P content and nutrient resorption (Woods et al. 2003; Vergutz et al. 2012; He et al. 2020). In several studies of plant adaptations to global warming (with differences between temperature treatments of 2–5°C), the average decrease in foliar nutrient content was about 3% for both N and P at the higher temperature (Chapin et al. 1995; Michelsen et al. 1996). Likewise, low-elevation plants usually exhibit a lower N content than their high-elevation conspecifics (Körner 1989). A higher N and P leaf content can partly compensate for decreases in the metabolic rate at low temperatures (Woods et al. 2003). Additionally, the growth rate of new tissues (leaves) may be directly limited by low temperatures at higher elevations, leading to a smaller sink for nutrients and their higher content in mature leaves (Körner 2003).
Until now, there have been only few studies (Nordell and Karlsson 1995; Dorrepaal et al. 2005; Aerts et al. 2007; Vergutz et al. 2012; Gerdol et al. 2019) on possible effects of other environmental factors, such as temperature change, on nutrient resorption efficiency and proficiency, as the main focus has usually been paid to their nutritional regulation (e.g. Aerts 1996; Killingbeck 1996; Aerts et al. 1999; Güsewell 2005b; Xu et al. 2020). In a high-latitude peatland, the experimental spring warming reduced senesced leaf N content in Vaccinium uliginosum (i.e. reduced nutrient resorption proficiency) whereas summer warming led to its reduction both in Betula nana and Rubus chamaemorus during a five-year warming experiment (Aerts et al. 2007). However, the experimental warming did not change REN, probably due to the small overall increase of mean air temperature (by only 0.9°C). On the other hand, a significantly lower REN with increasing summer temperature was reported in Betula pubescens subsp. tortuosa trees at 380 m a.s.l. compared to trees growing at 670 m a.s.l. in northern Sweden (Nordell and Karlsson 1995). Similarly, a significant decrease of both REN and REP with increasing mean air temperature was reported by Vergutz et al. (2012) along a latitudinal gradient in their global meta-analysis.
Central European temperate peatbogs are rather scattered, and many of them have been negatively impacted by drainage, peat extraction or eutrophication in the past (Rybníček et al. 2017). Nevertheless, the mountain peatbogs still harbour typical biodiversity, which can be negatively influenced by the ongoing climatic change. Changes in leaf nutrient contents in dominant plant species and both nutrient resorption efficiency and proficiency (i.e. leaf litter nutrient content) may have an important feedback on nutritional plant–soil interactions (e.g. leaf litter decomposability, Ward et al. 2010) and peatbog functioning (Dorrepaal et al. 2005). To study these topics, we used an elevational gradient as a spatial analogue for future increases in temperature using a space-for-time approach (cf. Dorrepaal et al. 2005). We compared the foliar N and P resorption efficiencies and proficiencies of two co-occurring typical dominant plant species (Molinia caerulea and Vaccinium uliginosum) at four microsites in each of two partially drained and extracted peatbogs in southern Bohemia, Czechia, at two different elevations. The two study sites have a similar history, soil-nutrient conditions and water table fluctuation, but differ in mean air temperature and growing season length. The mean air temperature difference (2°C) between the lowland and the mountain peatbog corresponds well to the anticipated global warming scenario for this part of Central Europe until 2050 (Ministry of the Environment of the Czech Republic 2017). In accordance with the literature, we hypothesized (1) an elevational increase in both mature and senescent leaf nutrient contents (i.e. nutrient proficiency) and (2) an increase in N and P resorption efficiency. Additionally, we studied whether the local N and P contents in the soil are related to foliar N and P contents in mature and senescent leaves or have an impact on N and P resorption efficiencies.
Material and Methods
Site Description
The study was conducted in two peatlands at different elevations in southern Bohemia, Czechia: The lowland Kozohlůdky (KZ) peatbog near Soběslav is part of the peatland complex of Borkovická blata at 410 m a.s.l. representing a special area of conservation, and the mountain Kapličky (KP) peatbog Nature Reserve near Vyšší Brod is located at 900 m a.s.l. (for more site characteristics, see Table 1). Both sites are characterized by very acidic peaty soils (pH 3.4–3.8; Table 2). The sites differ in their mean summer as well as annual temperatures by ca 2°C. At each site, four different experimental microsites were chosen which represented typical peatbog herb and low shrub vegetation. The four microsites were very similar in their biotic and abiotic conditions and can be accepted as replicates. Plant dominants within typical terrestrial peatbog communities included Molinia caerulea, Eriophorum angustifolium, Eriophorum vaginatum, Vaccinium uliginosum, Carex rostrata, Carex lasiocarpa and Carex nigra at both sites. The monocot graminoid M. caerulea (Poaceae) and the dicot shrub V. uliginosum (Ericaceae) were selected for this mineral nutritional study because they represent dominant plant species in Central European peatlands. They are relatively eurytopic and occur in habitats of several types, including peatbogs, fens and wet forests. Both have seasonal (short span, deciduous) leaves, and also have commonly been used for similar studies (e.g. Aerts 1989; Aerts and Caluwe 1989; Güsewell and Koerselman 2002; Güsewell 2005b; Xu et al. 2020).
Leaf Sampling
To determine N and P resorption efficiency and proficiency in above-ground biomass, green, fully developed, mature leaves were collected from four plant individuals of M. caerulea and V. uliginosum at each of the four microsites (the size of the individual microsites corresponded to ca 25 m2) at both sites in mid-August 2020, that is, before the end of the growing season. Two segments ca 4 cm long (one sample) were excised using scissors from the central part of one mature leaf of four M. caerulea plants at each microsite and stored separately (a total of 32 samples from 32 plants at both sites). Simultaneously, three to four mature leaves of V. uliginosum were sampled using forceps from one longer branch of each of four plants (a total of 48–64 leaves from 16 plants): They were sampled from the two-year twig just below the place of sprouting of the young, one-year twig. Similar sampling of senescent leaves of both species at both sites and all microsites was conducted on 20 October 2020 at KP and on 21–27 October 2020 at KZ due to differences in V. uliginosum phenology. Special attention was paid to the selection of similar age categories of mature and senescent leaves (van Heerwaarden et al. 2003). At both sites, we collected senescent leaves shortly after the first frosts had come, when completely yellow-brown M. caerulea leaves and dark-red V. uliginosum leaves were thought to be fully senescent. To measure specific leaf area (SLA; in m2 · kg−1) for resorption efficiency estimation, ca 12 similar leaf segments of M. caerulea and 25 leaves of V. uliginosum were sampled from different plants at one representative microsite both at KZ and KP at both summer and autumnal sampling times.
Analytical Procedures
The collected leaves were transported to the laboratory the same day and dried at 80°C to a constant dry weight (DW) for 6–8 h. Approximately 1–1.6 mg DW from one leaf (only the lamina) of V. uliginosum or one leaf segment of M. caerulea were weighed for N estimation and 2.1–3.5 mg DW from the same organ for P estimation. For N, the material was mineralized by concentrated H2SO4 at 310°C for 8 h and, for P, by concentrated HClO4 at 185°C for 3 h, then diluted and analysed for N and P content colourimetrically by an automatic FIAstar 5010 Analyzer (Tecator, Sweden; see Adamec 2002). Leaf area was scanned using a desk scanner and the program ImageJ to calculate the SLA. Then the leaves were dried at 80°C to a constant DW and weighed to determine the SLA. The decrease in SLA as the determination of autumnal loss of leaf biomass due to ageing and resorption, was used as a mass loss correction factor (MLCF) for the estimation of N and P resorption efficiency (see e.g., Güsewell 2005b; Vergutz et al. 2012). Values of REN (in %) were calculated as the relative loss of N pools in leaf tissues in senesced leaves according to equation (1). An analogical equation was used for the calculation of REP.
where N (or P)mat and N (or P)sen is the mean nitrogen (or phosphorus) content in mature and senesced leaves at individual microsites on a mass basis, and MLCF is the mass loss correction factor, specifically the ratio of the dry mass of senesced leaves and that of mature leaves.
Resorption proficiency was equal to the estimated analytical N or P content in senesced leaves (Killingbeck 1996). In a few samples of senesced leaves of both species from both sites, both N and P contents were unexpectedly high. Although the age category and colour of these leaves were the same as in other leaves collected from neighbouring plants at the same microsite and the V. uliginosum leaves started dehiscing, this may indicate that the N and P resorption in these leaves was delayed. These data were discarded and other material from the same plant was used for analyses. Values of foliar N and P content are expressed as percentages of DW and N : P ratios are presented on a mass basis, as usual.
Soil Sampling
Five subsurface peat soil samples (5–10 cm deep, approx. 100 g each) were collected together with the leaf samples from all experimental microsites in October 2020. The soil subsamples were homogenized within each microsite. A subsample of 15 g wet soil was dried at 60°C to measure the soil moisture content. In the dry soil sample, loss of weight (i.e. the content of organic matter) by combustion at 550°C was estimated. Water pH was measured in wet soil samples. NH4+-N, NO3−-N and PO4-P content in wet soil samples eluted by 1-M KCl (ISO/DIS 14 256-1) was measured using a FIAstar 5010 Analyzer.
Statistical Analysis
A Student t-test was used to compare soil nutrient contents (NH4+-N, NO3−-N, PO4-P) between the two sites. Differences in nutrient leaf contents (N, P) and N : P ratios between the study sites were tested using hierarchical nested ANOVA. Leaf contents from different individuals, nested in microsites (random factor), nested in the two peatlands (fixed factor) were used as explanatory variables. Differences in REN and REP between the two species and two sites were tested using two-way ANOVA. We quantified the linear regression models between REN, REP and mean nutrient contents in mature and senescent leaves and soils for each microsite using Pearson´s linear correlation coefficients (r) and present the data in a correlation matrix. We are aware of that several parameters (e.g. REN, N content in mature and senescent leaves, N : P content ratio) are more strongly or more weakly interrelated by definition. Where possible, all analytical data are stated as means ± SE at the four microsites. All statistical analyses were done in Statistica (version 6.0, StatSoft Inc. 2004).
Results
Peat soils at both experimental peatland sites, the lowland one at Kozohlůdky (KZ) and the mountain one at Kapličky (KP), had a very high mean organic matter content (90–93%) and very low pH values (3.55–3.77; Table 2, S1). The mean extractable NH4+-N content of only 6.8–8.1 mg · kg−1 exceeded that of NO3--N by about ten times, but the mean PO4-P content was in the range of 43–51 mg · kg−1. No statistically significant difference in these soil nutrients was found between the two sites.
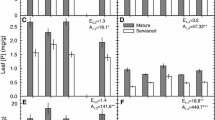
The N content in mature leaves of M. caerulea at KZ (2.36 ± 0.09%) was significantly higher than that at KP (2.00 ± 0.06%), but the higher foliar P content at KP was not significant (Table 3; Fig. 1). Thus, the foliar N : P ratio was higher at KZ, but the difference was only weakly significant. Both N and P content in senescent leaves was greatly reduced (Fig. 1) when compared to that in mature leaves at both sites, so the REN was 84–85% and the REP was 92–94% (Table 4). This very high REP caused the N : P ratio in senescent leaves to be 51–55.
In mature leaves of V. uliginosum, the N content was the same at both sites (Table 3, Fig. 1), and the higher foliar P content at KP was not significant (P = 0.062); the N : P ratio was thus lower at KP (18.8–28.3), but this difference was non-significant, too. By contrast, the P content in senescent V. uliginosum leaves was two times larger at KP than at KZ (P = 0.011). The N content in senescent leaves was only about 3–4 times lower than in mature ones (Table 3, Fig. 1). SLA increased in both species during the senescence (Table 3). The loss of foliar biomass due to senescence was 12.7–21.6% in M. caerulea and 13.2–15.1% in V. uliginosum at both sites. In summary, REN (70–78%) and REP (61–70%) were significantly lower for V. uliginosum than those in M. caerulea (Table 4). In both species, the content of neither N nor P in mature leaves correlated with extractable inorganic nutrients in the peaty soil (Tables 5, 6). The mature N and P leaf contents did not correlate with each other, either. Only the N content in senescent leaves of M. caerulea correlated negatively with the soil content of PO4-P. Also, a negative relationships between the N : P ratio in senescent leaves and the content of P in mature leaves of V. uliginosum was found. In both species, neither REN nor REP correlated with the inorganic nutrient content in the soil.
Discussion
Comparisons of nutrient resorption in the same species along an elevational gradient, besides the warming experiments, can be used as a proxy for the effect of ongoing climatic change, as both temperature and growing season length are changed synchronously across the whole study sites. With regard to the soil chemistry, the two sites used in this study were very similar and the nutrient content differences between them were at a maximum of 26% (Table 2, Table S1 in the Electronic supplementary material). The ammonium form of N strongly prevailed over the nitrate N at all microsites, indicating anoxic soil conditions typical of the peatlands. The soil N : P ratio of extractable N and P was very low at both peatland sites, which is in contrast with the relatively high N content in mature leaves (1.93–2.36%) found in both species and demonstrates efficient N uptake from the peaty soils. The foliar P content lay within the range of 0.073–0.115% in both species, and together with that of N, it is similar to values reported for both species or other peatland deciduous shrubs or graminoids in the literature (cf. Aerts 1989, 1996; Aerts et al. 1999; Güsewell and Koerselman 2002; Güsewell 2005b; Xu et al. 2020). As opposed to most literature cited, the N and P contents of mature leaves did not correlate with each other (Tables 5, 6). Moreover, N and P contents in mature leaves did not correlate with any of the soil chemistry parameters (Tables 5, 6). This is due to the fact that our elevational gradient was rather short (400–900 m a.s.l.) whereas significant correlations are more likely found along larger elevational / latitudinal gradients (Dorrepaal et al. 2005; Gerdol et al. 2019). Both lowland and mountain study sites are relatively large, therefore, the selected microsites differed slightly in the mean ground water table. However, no relationship of the results with the ground water table was found (data not presented).
The average mature leaf N : P mass content ratios were higher in both species than common values reported for terrestrial plants at natural sites (i.e. 12–13 in Güsewell and Koerselman 2002). In particular, the higher foliar N : P ratio in both species at KZ (26.1–28.3) could indicate a P limitation (Koerselman and Meuleman 1996; Güsewell and Koerselman 2002; Güsewell et al. 2003; Güsewell 2004). However, the concept of N or P growth limitation is based on short-term fertilization experiments and should not be automatically applied to plants in natural habitats, where other growth co-limitations occur (e.g. Güsewell and Koerselman 2002; Güsewell et al. 2003; Güsewell 2004).
In contrast to our expectation, we found an elevational decrease in mature leaf N content in M. caerulea. We found only a non-significant elevational nutrient content increase in mature leaf P content in V. uliginosum, but no other significant differences in mature leaf nutrient content along the elevational gradient of 500 m. Higher values of leaf nutrient contents are regularly found in alpine plants at high elevations (Körner 1989). The greatest leaf N pool is RuBP-carboxylase-oxydase in the leaves of C3 plants, directly involved in the leaf metabolism (Huffaker 1982); its higher concentration may therefore compensate for lower metabolic rate under low-temperature conditions. However, the temperature conditions are not so limiting for the growth of the two study species in our mountain peatbog during the growing period (mean July temperature of 15–16°C, see Table 1). Thus, the significant increase of leaf nutrient content along the elevational gradient would be manifested more strongly in more extreme conditions (high latitudes, the alpine zone above the treeline, etc.). Aerts et al. (2007) found no effect of either mild summer or winter warming on mature leaf N content in V. uliginosum, a species well adapted to high latitudes, either.
Similarly, nitrogen resorption proficiency (i.e. N content in senescent leaves, Fig. 1) did not change in either species along the elevational gradient, similar to the P resorption proficiency in M. caerulea (Table 3). According to our expectation, the P resorption proficiency increased with elevation in V. uliginosum, probably as a result of the non-significant elevational increase of P content in mature leaves and the weak correlation of both values to the soil PO4-P content (Table 6). The very low nutrient proficiency (i.e. low N and P content in senescent leaves), especially in M. caerulea, was close to the values in Sphagnum sp. div. litter (e.g. Hájek and Adamec 2009). Nevertheless, Certini et al. (2015) reported faster litter decomposition for M. caerulea and Calluna vulgaris in comparison with Sphagnum litter.
Although all senescent leaves of both species sampled within each site and microsite were from the same age category of leaves and had the same colour and, moreover, V. uliginosum leaves started dehiscing from the branches, the results proved that in a minor proportion of leaves, even within the same plant in both species and at both sites, N and P resorption were delayed. It is not clear why the resorption process is asynchronous within a plant micropopulation and also within single plants, but this fact should be taken into account in similar studies. Several authors (e.g. Nordell and Karlsson 1995) mentioned that the most appropriate way to study resorption efficiencies might be using of the same leaves for repeated measurements of their nutrient content due to large infraspecific leaf nutrient content variability. The recent use of near infrared spectroscopy to estimate nutrient contents in individual leaves without destructive sampling (e.g. Bon et al. 2020) might allow to researchers to apply this approach in the near future.
No elevational difference in REN or REP was found in any of the species examined in this study, indicating that the resorption efficiencies of neither species will change markedly under the projected increase in temperature and the length of the growing season in the mountain peatbog (at 900 m a.s.l.). By contrast, both N and P resorption efficiencies increased between 1,200 and 2,200 m a.s.l. in the Italian Alps (i.e. along 1,000-m wide elevational gradient; Gerdol et al. 2019). They found that the main process regulating nutrient resorption was a negative feedback effect on nutrient availability in the mineral soil, especially on the contents of organic N forms for nitrogen resorption efficiency (REN) and of inorganic P forms for phosphorus resorption efficiency (REP). The estimation of organic N and P soil content might be advantageous also in our study sites with highly organic soils, where organic forms are probably more available and more important for plant nutrition than inorganic forms. Therefore, the absence of a correlation of both mature leaf N contents and resorption efficiencies with soil inorganic N content was probably affected by the absence of such data in our study.
Both species exhibited very efficient N and P resorption from senescent leaves (Table 4): In M. caerulea, the REP exceeded the REN by ca 7–10%, while the opposite relationship between these parameters applied for V. uliginosum. These results also agree with abundant literature data on foliar REN and REP values for the same species or for plants growing especially at mineral-poor wetland sites (Aerts 1989, 1996; Aerts and Caluwe 1989; Aerts et al. 1999; Güsewell and Koerselman 2002; Güsewell 2005b; He et al. 2020; Xu et al. 2020). Very high foliar REN and REP values were reported for wetland graminoids by Güsewell (2005b), who concluded that better nutrient economy played a greater role for graminoids with shorter leaf life span in comparison with deciduous trees. We found larger intraspecific differences in both REN and REP in V. uliginosum than in M. caerulea (see the SE intervals in Table 4). Large differences in REN between individual trees were found also in Betula pubescens subsp. tortuosa (Nordell and Karlsson 1995).
On the basis of extensive literature data on 894 terrestrial plant species, comprising 2,541 records, He et al. (2020) determined the REN : REP ratio for all species to be 0.88, with small variations for single functional plant groups, which corresponds to the ratios determined for M. caerulea in this study (0.93 and 0.89 at the lowland and the mountain site, respectively). Thus, M. caerulea reabsorbed P from senescent leaves more effectively than N; the opposite pattern was found in V. uliginosum. Therefore, the two species use different strategies of leaf N and P resorption (cf. Güsewell 2005b; Xu et al. 2020). The observed high REP and REN in M. caerulea could contribute to the dominance of this species in P-limited and non-managed peatlands, as generally suggested for graminoids and stress tolerators (e.g. Güsewell 2004, 2005b). Similarly, the study by Xu et al. (2020) concludes that non-mycorrhizal graminoid species increase their fitness by minimizing nutrient losses through high nutrient resorption rather than by promoting nutrient uptake through mycorrhizal symbioses. By contrast, V. uliginosum is a shrub with an ericoid mycorrhiza and this fact can also elucidate its different N and P resorption strategy (Xu et al. 2020).
In line with literature data, the peatland dominants M. caerulea and V. uliginosum exhibit different strategies of N and P resorption from senescing leaves. The high REN and REP values observed in M. caerulea obviously underlie its strong dominance in both unmanaged peatbogs affected by drainage in the past. On the basis of our comparison of resorption efficiencies at the lowland and mountain sites, it is possible to presume that the nutrient resorption efficiencies and proficiencies of neither of the two species within the mountain population (at 900 m a.s.l.) will change markedly under the projected increase in temperature and growing season length. However, given the large interspecific differences in nutrient resorption traits (Aerts et al. 2007), substantial changes may be expected in nutrient cycling in temperate mountain peatlands after changes in the species composition, for example due to an increase of the dominance of deciduous trees (Betula pendula, Frangula alnus, Salix aurita) as recently observed at the lowland study site (A. Kučerová, unpubl. data).
References
Adamec L (2002) Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytol 155:89–100
Aerts R (1989) Aboveground biomass and nutrient dynamics of Calluna vulgaris and Molinia caerulea in a dry heathland. Oikos 56:31–38
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608
Aerts R, Caluwe H de (1989) Aboveground productivity and nutrient turnover of Molinia caerulea along an experimental gradient of nutrient availability. Oikos 54:320–324
Aerts R, Cornelissen JHC, van Logtestijn RSP, Callaghan TV (2007) Climate change has only a minor impact on nutrient resorption parameters in a high-latitude peatland. Oecologia 151:132–139
Aerts R, Verhoeven JTA, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80:2170–2181
Bon P, Bohner H, Kaino S, Moe T, Brathen KA (2020) One leaf for all: chemical traits of single leaves measured at the leaf surface using near-infrared reflectance spectroscopy. Meth Ecol Evol 9:1061–1071
Certini G, Vestgarden LS, Forte C, Strand LT (2015) Litter decomposition rate and soil organic matter quality in a patchwork heathland of southern Norway. Soil 1:207–216
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Dorrepaal E, Cornelissen JHC, Aerts R, Wallen B, Van Logtestijn RSP (2005) Are growth forms consistent predictors of leaf litter quality and decomposability across peatlands along a latitudinal gradient? J Ecol 93:817–828
Gerdol R, Iacumin P, Brancaleoni L (2019) Differential effects of soil chemistry on the foliar resorption of nitrogen and phosphorus across altitudinal gradients. Funct Ecol 33:1351–1361
Güsewell S (2004) N : P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Güsewell S (2005a) High nitrogen: phosphorus ratios reduce nutrient retention and second-year growth of wetland sedges. New Phytol 166:537–550
Güsewell S (2005b) Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct Ecol 19:344–354
Güsewell S, Koerselman W (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect Plant Ecol 5:37–61
Güsewell S, Koerselman W, Verhoeven JTA (2003) N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol Applic 13:372–384
Hájek T, Adamec L (2009) Mineral nutrient economy in competing species of Sphagnum mosses. Ecol Res 24:291–302
He M, Yan Z, Cui X, Gong Y, Li K, Han W (2020) Scaling the leaf nutrient resorption efficiency: nitrogen vs phosphorus in global plants. Sci Total Environm 729:138920
Heerwaarden LM van, Toet S, Aerts R (2003) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101:664–669
Huffaker RC (1982) Biochemistry and physiology of leaf proteins. In: Boulter D, Parthier B (eds) Encyclopedia of plant physiology, vol 14A. Springer-Verlag, Berlin, pp 370–400
ISO/DIS 14 256-1 Soil quality – Determination of nitrate, nitrite and ammonium in field moist soils by extraction with potassium chloride solution – Part 1: Manual method. International Organization for Standardization
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Koerselman W, Meuleman AF (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Körner C (1989) The nutritional status of plants from high altitudes. A worldwide comparison. Oecologia 81:379–391
Körner C. (2003). Mineral nutrition. In: Alpine plant life. Springer, Berlin, Heidelberg. pp 149–169
Michelsen A, Jonasson S, Sleep D, Havström M, Callaghan TV (1996) Shoot biomass, δ13C, nitrogen and chlorophyll responses of two arctic shrubs to in situ shading, nutrient application and warming simulating climatic change. Oecologia 105:1–12
Ministry of the Environment of the Czech Republic (2017) Seventh national communication of the Czech Republic under the United Nations framework convention on climate change including supplementary information pursuant to article 7.2 of the Kyoto Protocol. Available at https://unfccc.int/files/national_reports/annex_i_natcom_/application/pdf/17589243_czech_republic-nc7-br3-1-nc7_br3_cze.pdf (Accessed 8 February)
Nordell KO, Karlsson PS (1995) Resorption of nitrogen and dry matter prior to leaf abscission: variation among individuals, sites and years in the mountain birch. Funct Ecol 9:326–333
Rybníček K, Navrátilová J, Bufková I, Kučerová A (2017) Czech Republic. In Joosten H, Tanneberger F, Moen A (eds), Mires. Schweizerbart, Stuttgart, pp 341–351
Tolasz et al. (2007) Climate atlas of Czechia. Czech Hydrometeorological Institute, Prague, 254 pp [in Czech]
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220
Ward SE, Ostle NJ, McNamara NP, Bardgett RD (2010) Litter evenness influences short-term peatland decomposition processes. Oecologia 164:511–520
Woods HA, Makino W, Cotner JB, Hobbie SE, Harrison JF, Acharya K, Elser JJ (2003) Temperature and the chemical composition of poikilothermic organisms. Funct Ecol 17:237–245
Xu JW, Lin G, Liu B, Mao R (2020) Linking leaf nutrient resorption and litter decomposition to plant mycorrhizal associations in boreal peatlands. Pl & Soil 448:413–424
Acknowledgements
This study was partly supported by the Long-term research development project RVO 67985939 and by the Interreg AT-CZ45 project. Sincere thanks are due to Dr Brian G. McMillan (Glasgow, Scotland) for language corrections and to Tomáš Kučera for the statistical consultation. Special thanks are due to our colleagues Mrs Hana Strusková and Mrs Andrea Zajíčková (Chemical Laboratory, Institute of Botany of the Czech Academy of Sciences, Třeboň, Czechia) for skilful chemical analyses and to two anonymous referees for valuable comments improving the manuscript. The permission to sample and work in both protected areas was kindly provided by the Regional Authority of the South Bohemian Region in České Budějovice.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kučerová, A., Adamec, L. Foliar Resorption Efficiency Does Not Change Along an Elevational Gradient in Two Dominant Peatbog Plant Species. Folia Geobot 57, 247–257 (2022). https://doi.org/10.1007/s12224-023-09427-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-023-09427-4