Abstract
Plant succession is a fundamental process of vegetation recovery on disturbed sites. Elucidating its mechanisms remains a challenge as succession is influenced by stochastic and deterministic processes related to abiotic and biotic filters. Here, we use a multifaceted diversity approach to reveal mechanisms of successional changes in European oak-hornbeam forests during the first 10 years after selective logging. As the mechanisms controlling succession may depend upon initial abiotic conditions and colonization potential of the surrounding vegetation, we compare changes in taxonomic, functional, and phylogenetic diversity between clearings connected with open habitats and those isolated inside forests. Despite fewer dispersal barriers and higher biomass production in connected clearings, similar mechanisms initially governed succession in post-logging sites. Both clearings had low taxonomic and functional diversity in the first year of succession, as evidenced by significant trait convergence, caused by the legacy of interactions between overstory and understory vegetation in pre-disturbance closed-canopy forests. Colonization by short-lived and light-demanding species in the second and third years after logging has markedly increased the overall taxonomic and functional diversity, as evidenced by significant trait divergence. Connected clearings had higher functional but lower taxonomic and phylogenetic diversity than isolated clearings from the fourth to ten years of succession, probably due to intense competition in more productive habitats. All diversity facets markedly decreased in the last years due to increasing asymmetric competition from regenerating trees. The successional processes were largely deterministic, driven by species’ life-history strategies and biotic interactions (competition) rather than abiotic constraints and stochastic events.
Similar content being viewed by others
Introduction
Plant succession, i.e., development of vegetation after disturbance, is a fundamental process of community and restoration ecology (Prach and Walker 2020). However, elucidating its mechanisms remains a challenge as succession is influenced by both deterministic and stochastic processes (Kreyling et al. 2011; Purschke et al. 2013; Batalha et al. 2015). Plant succession can be controlled by abiotic environmental and biotic filters, such as competition and facilitation (Huston and Smith 1987; Zanini et al. 2006; Jing et al. 2015). In addition, stochastic processes associated with rare events of dispersal, colonization, and extinction can significantly alter the course of plant succession (Prach and Walker 2020). Deterministic and stochastic processes usually work simultaneously and produce patterns that are difficult to discern only from changes in species composition.
Contrasting mechanisms acting during the succession can be inferred from plant strategies of co-occurring species (Götzenberger et al. 2012; Mudrák et al. 2016). These can be approximated by differences in plant traits related to dispersal, growth, and resource acquisition (Hedwall and Brunet 2016). The significance of deterministic processes, such as environmental filtering and biotic interactions, can be assessed by comparing the trait structure of target communities with random assemblies that could potentially be generated from the local species pool by stochastic processes (Mason et al. 2011). Strong environmental filters often create communities where species are more similar to each other than expected by chance (Mayfield and Levine 2010). Early successional stages often face harsh environmental conditions that tend to select similar species that have converged in their ability to reach a new site and exploit its limited resources (Dolezal et al. 2008). In contrast, less environmentally demanding later succession phases select plants with different spatiotemporal niches (divergent strategies in resource acquisition and use) to avoid intense competition in more productive habitats (MacArthur and Levins 1967; Galland et al. 2019; Backhaus et al. 2021; de Bello et al. 2021).
Changes in trait dispersion patterns during succession, from convergence to divergence, can, therefore, reveal contrasting assembly mechanisms (equalizing versus stabilizing ones, Chesson 2000). However, care must be taken when inferring community assembly rules because different processes may produce the same trait dispersion patterns (Weiher and Keddy 1995). For instance, besides environmental filtering, intense asymmetric competition for light in later-successional communities leads to trait convergence (Mayfield and Levine 2010; Joner et al. 2012), while facilitation in harsh conditions can lead to trait divergence (Beltrán et al. 2012; Dolezal et al. 2019). Additional caution should be taken when assembly mechanisms are derived using a comparative (chronosequence) space-for-time substitution approach (Feldpausch et al. 2007; Johnson and Miyanishi 2008), or a few snapshots separated by long time intervals (Hédl et al. 2010; Kapfer et al. 2017). In that case, there is usually no information about underlying site-specific conditions or compositional changes ongoing in between snapshots.
The pitfalls associated with using chronosequence or snapshot data to derive assembly processes from trait dispersion patterns can be overcome in long-term successional studies using permanent monitoring plots within the framework of manipulative experiments that are designed to determine the effects of different conditions for vegetation development (Vild et al. 2013; Hédl and Chudomelová 2020). Studies combining experimental manipulation with annually resolved observations on taxonomic and trait composition are especially useful when successional turnover is rapid, such as secondary post-fire or post-logging forest succession in temperate climates (Meiners et al. 2015; Lanta et al. 2019; Kozel et al. 2021). In such conditions, where most resources and diaspores are readily available, environmental filtering can control succession only briefly, as short-lived pioneer species (e.g., annuals and biennials) are soon replaced by perennial herbs (Lanta et al. 2019), and this would be reflected in a rapid shift from trait convergence to trait divergence. However, the fast recovery of shrubs and trees can cause intense asymmetric competition for light that result in trait convergence again.
Annually resolved species abundance and trait dispersion data can, therefore, provide relatively precise information on the onset, duration, and extant of particular community assembly mechanisms during succession (Li et al. 2016). Yet it may remain unclear which traits are more influential and whether trait variation or the predominance of certain traits indicate contrasting assembly processes. Given these uncertainties, the trait dispersion patterns are better interpreted when combined with abundance-weighted mean trait values (de Bello et al. 2016; Götzenberger et al. 2016). For instance, strong environmental filtering related to drought and cold often forces all co-occurring species to converge to small stature with little size variation, while competition by tall dominants may increase both the trait mean and dispersion. Asymmetric competition can, however, increase the trait averages while reducing the variance as only tall species survive.
Finally, different traits may indicate different processes (Götzenberger et al. 2012; de Bello et al. 2021). Most studies focus on aboveground traits, such as plant height and leaf attributes that can best reflect competition for light. This may work in a productive environment where light is the most limiting factor (Mudrák et al. 2016). In less productive drier or colder places, belowground traits related to endurance, water and nutrient acquisition may better indicate contrasting assembly rules (Dolezal et al. 2019). In addition to measurable plant traits, the phylogenetic structure of successional communities can shade more light on assembly mechanisms (Webb et al. 2002; de Bello et al. 2017). Phylogenetic structure can capture adaptations that are not covered by measured plant traits, often those that have high phylogenetic conservatism reflecting a common evolutionary history of biotic interactions between closely related plants and their symbionts, herbivores, or pathogens (Craven et al. 2018). Early successional communities often consist of closely related taxa with good dispersal or nitrogen-fixing ability (e.g., Asteraceae or Fabaceae), while more phylogenetically diverse communities develop under more benign conditions of later-successional stages.
Here, we study which community assembly processes determine succession within the first 10 years in Central European species-rich oak-hornbeam forests, by comparing spatiotemporal changes in taxonomic, functional and phylogenetic diversity in contrasting forest clearings that differ in connectivity to open habitats. Because the mechanisms governing succession may depend to a large extent on the initial abiotic conditions, dispersal barriers, and colonization potential of the surrounding vegetation, we studied succession in connected and isolated clearings. Since connected clearings were opened to the forest edge and river corridors, we expected a more dynamic succession due to fewer dispersal barriers and higher biomass production leading to more intense competition and faster species turnover. In contrast, on isolated clearings located in enclosed forests, less dynamic successional changes were assumed due to more conservative conditions.
Specifically, we expected (1) the community assembly mechanisms on connected clearings to be initially less deterministic than on isolated clearings due to higher propagule pressure and more heterogeneous abiotic conditions, (2) stronger environmental filtering on isolated clearings with limited colonization and growth resulting in functional convergence, (3) greater functional divergence in connected clearings due to enhanced colonization and niche differentiation in a more productive and competitive environment, especially in mid-successional stages, (4) a clear trend towards functional and phylogenetic convergence between understory plants in later-successional stages due to increasing (asymmetric) competition for light from regenerating trees.
Materials and methods
Study area and management history
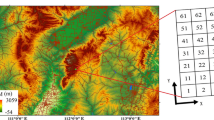
The experimental study was conducted in the canyon of Dyje river in Podyjí National Park (South Moravia, Czech Republic; 300 m a.s.l., 48°50′56″N, 15°53′13″E), which covers an area of 63 km2. Until the middle of the twentieth century, most of the area was managed as coppiced oak-hornbeam forests or wood pastures. This prevented the canopy from closing completely and created open forests with numerous mature trees. Traditional management has been abandoned over the last century, and forest canopy has been gradually closing and structurally homogenized due to secondary succession. The open forests became fragmented; today, they remained at the upper edge of the canyon.
Experimental design and data collection
Six pairs of clearings (~ 40 × 40 m) were created at six sites of the park core zone on the lower slopes of the Dyje river canyon (Fig. 1) in February 2011 (four pairs) and February 2012 (two pairs). Because there was another year of origin of the clearings, we refer to the “age of the clearing” in the following analyses and results. Several mature trees were left standing in the clearings to create conditions reminiscent of open forests (or coppice-with-standards woods). The original motivation for the introduction of openings in closed forests was the support of populations of endangered fauna and flora (e.g., critically endangered butterfly, Apollo Parnassius mnemosyne), the distribution of which is closely linked to the expansion of open stands.
One clearing of each pair was always connected to the alluvial meadow and adjacent forest edge (hereafter referred to as connected clearing) while the other was isolated from the meadow by at least a 20 m wide strip of dense forest (isolated clearing; Fig. 2). Vegetation data were collected every year from 2011 through 2012, 2013, 2014, 2015, 2016, 2018, and 2020 at the peak of the growing season in June. The cover of each plant species was visually estimated in 4–5 permanent plots 2 × 2 m in each 40 × 40 m clearing (see the list of recorded species in Table S1). In total, there were 50 permanent plots in both types of clearings. To study succession over years (Fig. 2), we kept a balanced design by combining data from 2018 (7 and 8-year-old clearings) and 2020 (9–10-year-old clearings).
a Connected clearing was opened to alluvial meadow and forest edge while isolated clearing was located inside the closed-canopy forest. Several retention trees were left within clearings. An example of the newly created clearing at the beginning of the experiment (b), after 5 years (c), and 10 years of succession (d)
Data analysis
To identify ecological differences between connected and isolated clearings, we retrieved Ellenberg’s indicator values (EIV; Ellenberg et al. 1992) of species affinity to light conditions, soil nutrients, moisture, and temperature. Six plant traits related to growth, resource acquisition, and seed dispersion (i.e., plant height [in m], specific leaf area [SLA (leaf area in mm2/leaf mass in mg)], leaf dry matter content [LDMC (100 × dry mass of leaf in mg/saturated mass of leaf in mg), seed weight [mg], terminal velocity [m/s2], and vegetative propagation (lateral spread in cm/year)] were selected to study the mechanisms that govern succession. The height of woody species was directly measured in the field every year while other traits were taken from databases (Kleyer et al. 2008; Klimešová et al. 2017).
We first calculated community-weighted EIV means (weighed by species percent cover in the community). Second, the community-weighted trait means (CWM; weighted by species percent cover in the community) for each plot were calculated separately for herb and woody plants. Functional diversity and phylogenetic diversity were calculated using mean pairwise dissimilarity (MPD; unrelated to species richness; de Bello et al. 2016). The MPD was used to calculate functional diversity in terms of the abundance-weighted mean of all pairwise (Gower’s) functional distances between community members, separately for communities of herb and woody species.
Whether the trait dispersion pattern is significantly divergent or convergent was tested by comparing the observed MPD with that of theoretical random communities. Significant trait divergence (higher MPD than expected by chance) refers to situations where coexisting species are more functionally dissimilar than expected at random, while convergence (lower MPD than expected by chance) refers to the situation when species are more similar than expected by chance. We built null models in which data randomization was performed within (1) each clearing by randomly assigning the species occurring in the clearing to the plots of that clearing, and (2) within the year. The number of species in the plot area of 2 × 2 m was maintained the same as in the field observation. In this respect, we computed the standardized effect size of functional traits (SES) for each plot as (MPDobs—MPDsim)/SDsim. The term MPDobs refers to the observed value of the MPD, MPDsim is the mean of the expected MPD and SDsim is the expected MPD standard deviation. The SES values reach zero when the null hypothesis is valid. Positive SES values indicate higher observed values than expected (“functional divergence”), while negative values indicate lower observed values than expected (“functional convergence”), and values close to zero mean random assembly pattern (Fig. 3; de Bello et al. 2016). Significant deviations of SES and SES PD values from zero were tested using a one-sample t test for each combination of clearing type and age. Additionally, we calculated phylogenetic diversity (PD) based on the dated ultrametric supertree of the European plant species “Daphne” (Durka and Michalski 2012), and for calculation, we used the abundance-weighted mean pairwise dissimilarity of all paired phylogenetic distances (MPD) between community members. The pairwise distances were calculated as cophenetic distances. The same approach (null model and randomization) described for FD was used to detect divergence/convergence in PD. The standardized effect size of phylogenetic diversity (SES PD) was then computed for each plot. Calculations of functional indices and dissimilarities were performed using R packages ‘FD,’ ‘picante,’ and ‘ape’ (R core team 2022).
Possible outcomes of the analyses concerning the magnitude of deviation between observed dissimilarity and generated values from null models. Error bars represent range of sample values (0.95% confidence intervals) on the Age/SES plot: functional divergence, functional convergence, and shift from random pattern to functional divergence
The effects of two clearing types, age, and their interaction on changes in species richness, EIV, CWM, SES, SES PD values and overall functional diversity (by Villéger et al. 2008), and phylogenetic diversity (MPD) were tested using Linear Mixed-Effects Models (LMM; R package ‘nlme’) with random effects (plot nested in site) to control for spatial heterogeneity and repeated measures scheme (repeated observations of individual plots). To visualize the linear (increase, decrease) and polynomial successional changes (quadratic or cubic term turning a linear regression into a curve) of one ‘hump’ (quadratic; a U or inverted U shape) or two ‘humps’ (cubic; one facing upward and the other down) in individual clearing types (connected vs. isolated), Generalized Additive Models (GAM) were run (R package ‘mgcv’; R core team 2022). Prior to the LMM analysis, the patterns in residuals were visually inspected in GAM plots and the scatter plots of residuals (Y axis) on the predictor (X axis—successional age). When the scatter plot showed patches of positive residuals in the center, but patches of negative residuals at either end (or vice versa), curved polynomial (quadratic or cubic) fit was applied in the LLM analysis (Fan and Gijbels 2013).
Results
Ecological conditions differed between connected and isolated clearings (Fig. 4). Connected clearings had higher soil nutrients and moisture, but lower temperatures than isolated clearings and no difference was found for light conditions. The richness and total cover of understory herb species showed a hump-shaped pattern over the 10 years of secondary succession in both clearing types (Fig. 5). There was an increase in richness and cover in the first 2 years, culmination in 3–4 years, and decrease in later years. In isolated clearings, herb species richness was higher and total cover was lower when compared to connected clearings (Table 1). The number of woody species was in general higher in isolated clearings (Table 1). Cover of woody species was low (below 20%) in the first four years but then markedly increased in both clearing types (Table 1). (Fig. 5). Functional diversity showed a hump-shaped pattern only in connected clearings and phylogenetic diversity fluctuated over succession similarly in both clearing types (Fig. 5).
Ecological characteristics of connected and isolated clearings, calculated as Ellenbergs’ indicator values (EIV) for nutrients, moisture, and temperature. Asterisks indicate significant differences between connected and isolated clearings (Liner Mixed-Effects Models; ***P < 0.001, *P < 0.05). Lines from GAM fit highlight the successional trends
Successional changes in species richness and percent cover of herb or woody species, and successional changes in functional, and phylogenetic diversities (herbs combined with woody species). Patterns are presented separately for connected (blue circles) and isolated clearings (orange circles). Means (circles) and confidence intervals (error bars) are shown. Asterisks indicate significant differences between connected and isolated clearings (Liner Mixed-Effects Models; *P < 0.05). Lines from GAM fit highlight the successional trends. (Color figure online)
The general trait dispersion pattern for herb species was convergent in the first year of succession, divergent between 2 and 6 years, and convergent again between 7 and 10 years. Not only trait dispersion (Fig. 6) but also community-weighted means (CWM) showed hump-shaped successional pattern (Fig. 7). In this respect, lower CWM was associated with convergent dispersion, while higher CWM was associated with divergent dispersion. However, there were some trait- and habitat-specific deviations from this general pattern (Figs. 6, 7).
Changes in trait dispersion (standardized effect sizes, SES) and phylogenetic diversity (standartized effect sizes of PD, SES PD) during succession, calculated for herb species. Error bars (0.95 confidence intervals) are displayed for every year and separately for connected (blue) and isolated (orange) clearings. Stars indicate significant deviation from zero based on one-sample t test; if significant, the position of error bars below zero line indicates convergence in a plant trait, SES or SES PD, while the position above the zero line indicates divergence. Lines from GAM fit highlight the successional trends. Note that the successional trends in connected or isolated clearings differed in SES for lateral spread and SES for LDMC. (Color figure online)
Changes in community-weighted trait means (CWM) during succession, calculated for herb species. Error bars (0.95 confidence intervals) are displayed for every year and separately for connected (blue) and isolated (orange) clearings. Lines from GAM fit highlight the successional trends. Note that the successional trends in connected or isolated clearings differed in CWM for SLA, CWM for LDMC, and CWM for lateral spread
The first year of succession displayed convergent patterns for most of the traits. Height, SLA, and seed weight were significantly more similar among coexisting herbs than random expectation (Table 1), regardless of the clearing type. The pattern of convergence in the first year of succession corresponded with the lowest mean trait values. Hence, the plants occurring on clearings the first year were on average shorter, with lower LDMC and seed weight, higher terminal velocity, and limited lateral spread (reduced clonality typical for short-lived taxa), and this pattern was common to most co-occurring species (not only dominants) as evidenced by their significant trait convergence. Unlike other traits, SLA in connected clearings was initially divergent with high mean values but convergent with low mean values in later years. In isolated clearings, SLA had a random dispersion pattern and more or less constant mean values over time.
Most of the traits among co-occurring herbs tended to diverge between the 2nd and 6th years. LDMC in both clearing types and lateral spread in connected clearings showed significant mid-succession divergence (Table 2, Fig. 6). While LDMC dispersion decreased with succession, mean LDMC values increased, the pattern driven by few late-successional dominants that increase community mean but reduce its variance. Most of the traits also tended to converge between the 7th and 10th years. Plant height and terminal velocity showed significant convergence in isolated clearings for the last year of observation (Table 2, Fig. 6).
For woody species, LDMC and lateral spread displayed the same hump-shaped dispersion pattern over the 10 years of succession as seen among herb species, from initial convergence to mid-succession divergence and late-succession convergence (Fig. S1). The opposite trend was found for woody species’ plant height, being significantly divergent after 4th year and in the last year, with a convergent pattern in between. In both herb and woody species, SLA was initially divergent but converged over time (significant in the last year). Seed weight did not show a clear trait dispersion pattern in herb and woody species, even though the mean seed weight decreased across succession. The lateral spread was significantly divergent in mid-succession in connected clearings for both herb and woody species (Tables 1, 2, Fig. S1). In isolated clearings, the lateral spread was significantly convergent in the early and later stages.
The phylogenetic structure among coexisting herb species was significantly divergent in both clearing types (Fig. 6), with higher phylogenetic diversity in isolated than connected clearings. There was only a temporary decline in phylogenetic diversity in the early stages (2nd and 3rd years), indicating that pioneer assemblages were composed of phylogenetically more similar species compared to random expectation (Fig. 6). Phylogenetic diversity was obviously much lower among woody species than among herbs (Fig. 6, Fig. S1). Phylogenetic structure tended to diverge over time (Fig. 6) among herbs colonizing connected clearings and to converge among woody species, especially within isolated clearings (Fig. S1).
Discussion
Our study examines the importance of contrasting community assembly rules based on the phylogenetic and functional structure of plant communities in connected and isolated clearings during the first 10 years of forest succession in Central European species-rich oak-hornbeam woodlands. Using a null model to distinguish between deterministic and stochastic processes, we have shown that the succession was largely a deterministic process controlled by biotic interactions rather than abiotic filtering (Purschke et al. 2013; Chang and HilleRisLambers 2019). We documented convergent development in the first year of succession reflecting the legacy of pre-disturbance forest conditions, random or divergent pattern between 2 and 6 years due to colonization by functionally dissimilar taxa, and again, the convergent development between 7 and 10 years due to asymmetric competition from regenerating tree layer. Biotic interactions generated both convergent and divergent patterns depending on the intensity and asymmetry of competition (mostly for light). Therefore, we do not confirm the expected unidirectional shift from convergence to divergence due to the change from abiotic filtering to biotic interactions (e.g., Backhaus et al. 2021). The last successional stage returned to trait convergence due to intensifying asymmetric competition for light from regenerating trees. This led to a decreasing taxonomic, functional, and phylogenetic diversity of the plant communities.
Initial years of post-logging succession
Convergent trait patterns in the first year of succession reflect the prevailing adaptations of typical forest understory species that remained in the newly created clearing from the original oak-hornbeam forests (Lanta et al. 2019). In both connected and isolated clearings, the plants were in overall shorter, with lower LDMC and limited clonal spread than expected by chance. The trait convergence observed in the first year of succession is, thus, determined by low light availability within the original forests (Vild et al. 2013) rather than by environmental filters on newly formed clearings represented by increased insolation, reduced humidity, or increased mineralization (Hédl et al. 2010; Verheyen et al. 2012). Therefore, functionally similar but phylogenetically diverse species adapted to closed-canopy conditions (shady and wet) predominate in the initial clearing communities, while the second and third-year communities are functionally more diverse mixtures of both original shade-tolerant taxa and newly occurring light-demanding pioneers, colonizing clearings. However, because most colonizing pioneers are recruited from only a few plant families (Asteraceae, Poaceae, Cyperaceae), the second and third-year communities have much lower phylogenetic diversity than the first-year communities.
Our expectation that the community assembly rules in the second and third year of succession will be less deterministic in connected than isolated clearings due to higher propagule pressure from the surrounding open landscape has not been fully supported by our data. We did not find stronger environmental filtering leading to functional convergence in isolated than connected clearings due to colonization barriers. In both connected and isolated clearings, colonization by short-lived herbs has shifted trait dispersion patterns from convergent to random or divergent as evidenced by increasing community mean and variance in plant height, SLA, and terminal velocity and decreasing seed weight (see also Batalha et al. 2015; Galland et al. 2019; Backhaus et al. 2021). The trait dispersion patterns in the second and third years are largely similar in both types of clearings, suggesting that colonization processes are similar regardless of location in the forest. However, because the colonizing pioneers are more phylogenetically similar in connected than isolated clearings, the isolated clearings have significantly higher phylogenetic diversity in the second and third years after logging.
Intermediate stages of post-logging succession
Our expectation that connected clearings will show greater functional divergence than isolated clearings, due to a higher level of colonization but also stronger competition, has gained support in the mid-successional stages between 4 and 6 years. Rapid colonization of connected clearings by clonal species at the expense of short-lived pioneers led to a more significant increase in phylogenetic and functional diversity compared to isolated clearings. It is known that the early stages of forest succession have more nutrients available through increased irradiation of the soil surface and consequent increased microbial activity and nitrogen mineralization (Binkley 1984; Verheyen et al. 2012). Such conditions stimulate the spread of light- and N-demanding short-lived pioneers as well as perennial taxa with greater dispersal and competitive abilities, such as grasses (Lanta et al. 2020). Bergholm et al. (2015) documented that the flash of N-demanding pioneer species does not last long (up to 5 years) because the period of increased nitrogen availability occurs immediately after the canopy is opened. This was seen in the connected clearings between the 2nd and 4th years. Clonal grasses, forbs, and shrubs from nearby alluvial meadows and forest edges soon accompany short-lived non-clonal pioneers to increase overall taxonomic, functional, and phylogenetic diversity in connected clearings.
The trait divergence observed between 3 and 6 years indicates niche differentiation (Mayfield and Levine 2010), with species coexisting rather than competing (Weiher and Keddy 1995; Mudrák et al. 2016). The limited niche overlap corresponds to the highest species diversity recorded in 10 years after logging. Higher functional diversity is associated with contrasting resource use strategies between co-occurring species in both above- and belowground, as evidenced by significant divergence in the LDMC and lateral spread (clonal growth strategies). Diversity in carbon gain strategies (leaf thickness), and soil nutrient and water acquisition by various roots and rhizomes, thus, allows the coexistence of a large number of taxa. However, functional divergence in the mid-successional stages may have been primarily associated with relatively high microhabitat variability (see also Keppel et al. 2017). Shrubs and trees resprouting from stumps, mixed with grasslands, anthills, and soil cavities support various plants that differ in ecological niches (Uotila and Kouki 2005). In addition, scarification of the soil surface caused by logging and a consequent decrease in moss cover is also reported to be beneficial for seed germination of early successional species of the ability to maintain long-term persistent soil seed banks (Pykälä 2004).
Later stages of post-logging succession
Our expectation that connected clearings will have a lower richness of coexisting species than isolated clearings due to higher overall productivity and more intense competition (Lepš 2014; Craven et al. 2018), has received support from the middle to late-successional stages between 3 and 10 years (see also Li et al. 2016). The importance of competition as the main assembly mechanism is indicated by the significant divergence of clonal growth strategies in connected but not in isolated clearings, together with higher productivity and lower taxonomic and phylogenetic diversity. Similar to the findings of other studies (Bartha et al. 2008; Galland et al. 2019), rather species with different ecological strategies may survive interspecific competition in productive habitats (Mudrák et al. 2016; Doležal et al. 2019), which maximizes divergence in traits but also reduces the number of coexisting species. However, when competition becomes intense and asymmetric, overall taxonomic, functional, and phylogenetic diversity may decline (Mayfield and Levine 2010; Loiola et al. 2018; Galland et al. 2019), as it was documented on isolated and connected clearings between 6 and 10 years of succession. In the last three years of observation, broadleaf tree species (especially common hornbeam Carpinus betulus and field maple Acer campestre) have dominated (Vild et al. 2013; Hédl and Chudomelová 2020). Their dense thickets with low light levels reduce the species richness of undergrowth herbs to the lowest levels, recorded during the 10 years of succession. Due to the impoverishment of species and phylogenetic richness, there is trait convergence in plant height and seed dispersal among remaining understory herbs, as well as a convergence in SLA and clonal propagation in woody species.
Conclusions
We studied community assembly mechanisms driving temperate forest succession after logging by comparing spatiotemporal changes in taxonomic, functional, and phylogenetic diversity between connected and isolated clearings. Non-random assembly mechanisms were driving the compositional changes in both connected and isolated clearings. Although they initially differed in dispersal barriers, colonization potential, biomass production, and successional rate, both were driven by similar mechanisms. Unlike other studies, trait convergence observed in the first year of succession was not the result of abiotic filtering on new clearings, but of the legacy of pre-disturbance forest and biotic interactions between canopy trees and understory herbs adapted to deep shade and moist soils. Colonization by short-lived forbs and light-demanding graminoids in the second and third years after logging has shifted trait dispersion patterns from convergent to random or divergent, and massively increased overall taxonomic, functional, and phylogenetic diversity. Connected clearings had higher functional but lower taxonomic and phylogenetic diversity than isolated clearings in the middle to late-successional stages because of more intense competition in more productive habitats and because the colonizing species were more phylogenetically similar in connected than in isolated clearings. Taxonomic, functional, and phylogenetic diversity of understory plants in later stages markedly decreased to the lowest levels, recorded during the 10 years of succession, due to increasing asymmetric competition for light from recovering maple and hornbeam trees.
Data availability
Data are available from Open Science Framework. Link: OSF|Functional and phylogenetic diversity across succession. Identifier: https://doi.org/10.17605/OSF.IO/PHQE2
References
Backhaus L, Albert G, Cuchietti A, Jaimes-Nino LM, Fahs N, Lisner A, Kolář V, Kermavnar J, Widmer S, Zimmermann Z, Rofrics N, de Bello F, Lepš J, Medina NG (2021) Shift from trait convergence to divergence along old-field succession. J Veg Sci 32:e12986. https://doi.org/10.1111/jvs.12986
Bartha S, Merolli A, Campetella G, Canullo R (2008) Changes of vascular plant diversity along a chronosequence of beech coppice stands, central Apennines, Italy. Plant Biosyst 142:572–583. https://doi.org/10.1080/11263500802410926
Batalha MA, Pipenbaher N, Bakan B, Kaligarič M, Škornik S (2015) Assessing community assembly along a successional gradient in the North Adriatic Karst with functional and phylogenetic distances. Oecologia 178:1205–1214. https://doi.org/10.1007/s00442-015-3295-5
Beltrán E, Valiente-Banuet A, Verdú M (2012) Trait divergence and indirect interactions allow facilitation of congeneric species. Ann Bot 110:1369–1376. https://doi.org/10.1093/aob/mcs089
Bergholm J, Olsson BA, Vegerfors B, Persson T (2015) Nitrogen fluxes after clear-cutting. Ground vegetation uptake and stump/root immobilisation reduce N leaching after experimental liming, acidification and N fertilisation. For Ecol Manag 342:64–75. https://doi.org/10.1016/j.foreco.2015.01.009
Binkley D (1984) Does forest removal increase rates of decomposition and nitrogen release? For Ecol Manag 8:229–233. https://doi.org/10.1016/0378-1127(84)90055-0
Chang CC, HilleRisLambers J (2019) Trait and phylogenetic patterns reveal deterministic community assembly mechanisms on mount st. Helens. Plant Ecol 220:675–698. https://doi.org/10.1007/s11258-019-00944-x
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Evol Syst 31:343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343
Craven D, Eisenhauer N, Pearse WD et al (2018) Multiple facets of biodiversity drive the diversity–stability relationship. Nat Ecol Evol 2:1579–1587. https://doi.org/10.1038/s41559-018-0647-7
de Bello F, Carmona CP, Lepš J, Szava-Kovats R, Pärtel M (2016) Functional diversity through the mean trait dissimilarity: resolving shortcomings with existing paradigms and algorithms. Oecologia 180:933–940. https://doi.org/10.1007/s00442-016-3546-0
de Bello F, Šmilauer P, Diniz-Filho JAF, Carmona CP, Lososová Z, Herben T, Götzenberger L (2017) Decoupling phylogenetic and functional diversity to reveal hidden signals in community assembly. Methods Ecol Evol 8:1200–1211. https://doi.org/10.1111/2041-210X.12735
de Bello F, Lavorel S, Hallett LM, Valencia E, Garnier E, Roscher C, Conti L, Galland T, Goberna M, Májeková M, Montesinos-Navarro A, Pausas JP, Verdú M, E-Vojtkó A, Götzenberger L, Lepš L (2021) Functional trait effects on ecosystem stability: assembling the jigsaw puzzle. Trends Ecol Evol 36:822–836. https://doi.org/10.1016/j.tree.2021.05.001
Dolezal J, Homma K, Takahashi K, Vyatkina MP, Yakubov V, Vetrova VP, Hara T (2008) Primary succession following deglaciation at Koryto Glacier Valley, Kamchatka. Arct Antarc Alp Res 40:309–322. https://doi.org/10.1657/1523-0430(06-123
Dolezal J, Dvorsky M, Kopecky M, Altman J, Mudrak O, Capkova K, Rehakova K, Macek M, Liancourt P (2019) Functionally distinct assembly of vascular plants colonizing alpine cushions suggests their vulnerability to climate change. Ann Bot 123:569–578. https://doi.org/10.1093/aob/mcy207
Doležal J, Lanta V, Mudrák O, Lepš J (2019) Seasonality promotes grassland diversity: Interactions with mowing, fertilization and removal of dominant species. J Ecol 107:203–215. https://doi.org/10.1111/1365-2745.13007
Durka W, Michalski SG (2012) Daphne: a dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93:E093-214. https://doi.org/10.1890/12-0743
Ellenberg H, Weber HE, Düll R, Wirth W, Werner W, Paulißen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa. . und verbesserte Aufkage. Scripta Geobotanica 18:1–248
Fan J, Gijbels I (2013) Local polynomial modelling and its applications. Monographs on statistics and applied probability. Springer, US
Feldpausch TR, Prates-Clark CDC, Fernandes EC, Riha SJ (2007) Secondary forest growth deviation from chronosequence predictions in central Amazonia. Glob Change Biol 13:967–979. https://doi.org/10.1111/j.1365-2486.2007.01344.x
Galland T, Adeux G, Dvořáková H, E-Vojtkó A, Orbán I, Lussu M, Puy J, Blažek P, Lanta V, Lepš J, de Bello F, Pérez Carmona C, Valencia E, Götzenberger L (2019) Colonization resistance and establishment success along gradients of functional and phylogenetic diversity in experimental plant communities. J Ecol 107:2090–2104. https://doi.org/10.1111/1365-2745.13246
Götzenberger L, de Bello F, Bråthen KA, Davison J, Dubuis A, Guisan A, Lepš J, Lindborg R, Moora M, Pärtel M, Pellissier L, Pottier J, Vittoz P, Zobel K, Zobel M (2012) Ecological assembly rules in plant communities—approaches, patterns and prospects. Biol Rev 87:111–127. https://doi.org/10.1111/j.1469-185X.2011.00187.x
Götzenberger L, Botta-Dukát Z, Lepš J, Pärtel M, Zobel M, de Bello F (2016) Which randomizations detect convergence and divergence in trait-based community assembly? A test of commonly used null models. J Veg Sci 27:1275–1287. https://doi.org/10.1111/jvs.12452
Hédl R, Chudomelová M (2020) Understanding the dynamics of forest understorey: combination of monitoring and legacy data reveals patterns across temporal scales. J Veg Sci 31:733–743. https://doi.org/10.1111/jvs.12882
Hédl R, Kopecký M, Komárek J (2010) Half a century of succession in a temperate oakwood: from species-rich community to mesic forest. Divers Distr 16:267–276. https://doi.org/10.1111/j.1472-4642.2010.00637.x
Hedwall P-O, Brunet J (2016) Trait variations of ground flora species disentangle the effects of global change and altered land-use in Swedish forests during 20 years. Glob Change Biol 22:4038–4047. https://doi.org/10.1111/gcb.13329
Huston M, Smith T (1987) Plant succession: life history and competition. Am Nat 130:168–198. https://doi.org/10.1086/284704
Jing J, Bezemer MT, van der Putten WH (2015) Complementarity and selection effects in early and mid-successional plant communities are differentially affected by plant-soil feedback. J Ecol 103:641–647. https://doi.org/10.1111/1365-2745.12388
Johnson EA, Miyanishi K (2008) Testing the assumptions of chronosequences in succession. Ecol Lett 11:419–431. https://doi.org/10.1111/j.1461-0248.2008.01173.x
Joner F, Anand M, Pillar VD (2012) Trait-convergence and divergence assembly patterns in a temperate forest herbaceous layer along the gradient of canopy closure. Community Ecol 13:178–184. https://doi.org/10.1556/ComEc.13.2012.2.7
Kapfer J, Hédl R, Jurasinski G, Kopecký M, Schei FH, Grytnes J-A (2017) Resurveying historical vegetation data—opportunities and challenges. Appl Veg Sci 20:164–171. https://doi.org/10.1111/avsc.12269
Keppel G, Anderson S, Williams C, Kleindorfer S, O’Connell C (2017) Microhabitats and canopy cover moderate high summer temperatures in a fragmented Mediterranean landscape. PLoS ONE 12:e0183106. https://doi.org/10.1371/journal.pone.0183106
Kleyer M, Bekker RM, Knevel IC et al (2008) The LEDA traitbase: a database of life-history traits of Northwest European flora. J Ecol 96:1266–1274. https://doi.org/10.1111/j.1365-2745.2008.01430.x
Klimešová J, Danihelka J, Chrtek J, de Bello F, Herben T (2017) CLO-PLA: a database of clonal and bud-bank traits of the central European flora. Ecology 98:1179. https://doi.org/10.1002/ecy.1745
Kozel P, Sebek P, Platek M, Benes J, Zapletal M, Dvorsky M, Lanta V, Dolezal J, Bace R, Zbuzek B, Cizek L (2021) Connectivity and succession of open structures as a key to sustaining light-demanding biodiversity in deciduous forests. J Appl Ecol 58:2951–2961. https://doi.org/10.1111/1365-2664.14019
Kreyling J, Jentsch A, Beierkuhnlein C (2011) Stochastic trajectories of succession initiated by extreme climatic events. Ecol Lett 14:758–764. https://doi.org/10.1111/j.1461-0248.2011.01637.x
Lanta V, Mudrák O, Liancourt P, Bartoš M, Chlumská Z, Dvorský M, Pusztaiová Z, Münzbergová Z, Sebek P, Čížek L, Doležal J (2019) Active management promotes plant diversity in lowland forests: a landscape-scale experiment with two types of clearings. For Ecol Manag 448:94–103. https://doi.org/10.1016/j.foreco.2019.05.073
Lanta V, Mudrák O, Liancourt P, Dvorský M, Bartoš M, Chlumská Z, Šebek P, Čížek L, Doležal J (2020) Restoring diversity of thermophilous oak forests: connectivity and proximity to existing habitats matter. Biodivers Conserv 29:3411–3427. https://doi.org/10.1007/s10531-020-02030-5
Lepš J (2014) Scale- and time-dependent effects of fertilization, mowing and dominant removal on a grassland community during a 15-year experiment. J Appl Ecol 51:978–987. https://doi.org/10.1111/1365-2664.12255
Li S-P, Cadotte MW, Meiners SJ, Pu Z, Fukami T, Jiang L (2016) Convergence and divergence in a long-term old-field succession: the importance of spatial scale and species abundance. Ecol Lett 19:1101–1109. https://doi.org/10.1111/ele.12647
Loiola PP, de Bello F, Chytrý M et al (2018) Invaders among locals: alien species decrease phylogenetic and functional diversity while increasing dissimilarity among native community members. J Ecol 106:2230–2241. https://doi.org/10.1111/1365-2745.12986
MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385. https://doi.org/10.1086/282505
Mason NWH, de Bello F, Doležal J, Lepš J (2011) Niche overlap reveals the effects of competition, disturbance and contrasting assembly processes in experimental grassland communities. J Ecol 99:788–796. https://doi.org/10.1111/j.1365-2745.2011.01801.x
Mayfield MM, Levine JM (2010) Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol Lett 13:1085–1093. https://doi.org/10.1111/j.1461-0248.2010.01509.x
Meiners SJ, Cadotte MW, Fridley JD, Pickett STA, Walker LR (2015) Is successional research nearing its climax? New approaches for understanding dynamic communities. Funct Ecol 29:154–164. https://doi.org/10.1111/1365-2435.12391
Mudrák O, Janeček Š, Götzenberger L, Mason NWH, Horník J, de Castro I, Doležal J, Klimešová J, de Bello F (2016) Fine-scale coexistence patterns along a productivity gradient in wet meadows: shifts from trait convergence to divergence. Ecography 39:338–348. https://doi.org/10.1111/ecog.01723
Prach K, Walker LR (2020) Comparative plant succession among terrestrial biomes of the world. Cambridge University Press, Cambridge, UK
Purschke O, Schmid BC, Sykes MT, Poschlod P, Michalski SG, Durka W, Kühn I, Winter M, Prentice HC (2013) Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: insights into assembly processes. J Ecol 101:857–866. https://doi.org/10.1111/1365-2745.12098
Pykälä J (2004) Immediate increase in plant species richness after clearcutting of boreal herb-rich forests. Appl Veg Sci 7:29–34. https://doi.org/10.1111/j.1654-109X.2004.tb00592.x
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 20 December 2022
Uotila A, Kouki J (2005) Understorey vegetation in spruce-dominated forests in eastern Finland and Russian Karelia: successional patterns after anthropogenic and natural disturbances. For Ecol Manag 215:113–137. https://doi.org/10.1016/j.foreco.2005.05.008
Verheyen K, Baeten L, De Frenne P, Bernhardt-Römermann M, Brunet J, Cornelis J et al (2012) Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J Ecol 100:352–365. https://doi.org/10.1111/j.1365-2745.2011.01928.x
Vild O, Roleček J, Hédl R, Kopecký M, Utinek D (2013) Experimental restoration of coppice-with-standards: response of understorey vegetation from the conservation perspective. For Ecol Manag 310:234–241. https://doi.org/10.1016/j.foreco.2013.07.056
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. https://doi.org/10.1890/07-1206.1
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Evol Syst 33:475–505. https://doi.org/10.1146/annurev.ecolsys.33.010802.150448
Weiher E, Keddy PA (1995) Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74:159–164. https://doi.org/10.2307/3545686
Zanini L, Ganade G, Hübel I (2006) Facilitation and competition influence succession in a subtropical old field. Plant Ecol 185:179–190. https://doi.org/10.1007/s11258-005-9093-0
Acknowledgements
We would like to express special thanks to administration of the Podyjí National Park, namely Lenka Reiterová, Robert Stejskal and Martin Škorpík, and our collaborators Zuzana Chlumská, Pierre Liancourt and Vít Pejcha.
Funding
Open access publishing supported by the National Technical Library in Prague. The study was financially supported by the Czech Science Foundation (21-26883S, 23-07533S), the Czech Academy of Sciences (RVO 67985939 and RVO 60077344), and MSMT INTER-EXCELLENCE project (LTAUSA18007).
Author information
Authors and Affiliations
Contributions
JD, PŠ, VL, and LČ conceived the ideas and designed methodology; JD, MD, MB, OM, and VL collected the data; VL and OM analyzed the data; VL and JD led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Yong-jian Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lanta, V., Mudrák, O., Dvorský, M. et al. Multifaceted diversity changes reveal community assembly mechanisms during early stages of post-logging forest succession. Plant Ecol 224, 335–347 (2023). https://doi.org/10.1007/s11258-023-01306-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-023-01306-4