Abstract
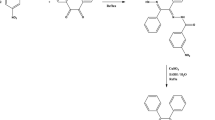
A new bidentate Schiff base ligand [HL = N-(2-hydroxy-1-naphthylidene)-2-methyl aniline] and its mononuclear Cu(II) complex with formula [CuL2] were prepared and characterized by Fourier-transform infrared (FT-IR), Nuclear Magnetic Resonance [1H and 13C{1H} NMR], Ultraviolet–visible (UV–Vis) spectroscopic methods, and elemental analysis. The Schiff base ligand [HL] and Cu(II) complex [CuL2] crystal structures were determined using the single crystal X-ray diffraction method. The data of crystal structure indicated a bidentate method of coordination for the Schiff base by NO donor sets in a regular square-planar fashion. The nano-sized [CuL2] was synthesized by the sonochemical method and was detected by X-ray powder diffraction (XRD), Field Emission Scanning Electron Microscopes (FE-SEM), and FT-IR spectroscopy. The average particle size in nano-sized [CuL2] was approximately 55 nm. The nano-complex [CuL2] was used in a modified screen-printed electrode (modified SPE) as an electrochemical sensor for the detection of 2-phenylphenol. The oxidation reaction of 2-phenylphenol at the modified electrode indicated a linear range of 0.01–600.0 µM and the recognition limit was 8.0 nM. The proposed electrode shows two well-separated oxidation signals for 2-phenylphenol and 4-chlorophenol, which makes it suitable for the simultaneous determination of these analytes. The fabricated sensor was successfully applied for the designation of 2-phenylphenol in lemon rind, orange rind, and water samples with satisfactory recovery.





















Similar content being viewed by others

References
A.A. Dehghani-Firouzabadi, F. Motevaseliyan, Synthesis and characterization of four new unsymmetrical potentially pentadentate Schiff base ligands and related Zn(II) and Cd(II) complexes. Eur. J. Chem. 5, 635–638 (2014)
E. Yousif, A. Majeed, Kh. Al-Sammarrae, N. Salih, J. Salimon, B. Abdullah, Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arab. J. Chem. 10, 639–644 (2017)
A. Sahraei, H. Kargar, M. Hakimi, M.N. Tahir, Synthesis, characterization, crystal structures, and biological activities of eight-coordinate zirconium (IV) Schiff base complexes. Transit. Met. Chem. 42, 483–489 (2017)
B.K. Singh, H.K. Rajour, A. Prakash, Synthesis, characterization, and biological activity of transition metal complexes with Schiff bases derived from 2-nitrobenzaldehyde with glycine and methionine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc 94, 143–151 (2012)
X. Zhong, J. Yi, J. Sun, H.L. Wei, W.S. Liu, K.B. Yu, Synthesis and crystal structure of some transition metal complexes with a novel bis-Schiff base ligand and their antitumor activities. Eur. J. Med. Chem. 41, 1090–1092 (2006)
T. Kondori, N. Akbarzadeh-T, M. Fazli, B. Mir, M. Dušek, V. Eigner, A novel schiff base ligand and its copper complex: synthesis, characterization, X-ray crystal structure and biological evaluation. J. Mol. Struct. 1226, 129395 (2021)
S. Madani, K. Mokhnache, A. Rouane, N. Charef, Synthesis, characterization and in vitro evaluation of antibacterial and antifungal activities of new Schiff base and its metal complexes. Mater. Biomater. Sci. 3, 001–009 (2020)
E. Ravi Krishna, P. Muralidhar Reddy, M. Sarangapani, G. Hanmanthu, B. Geeta, K. Shoba Rani, V. Ravinder, Synthesis of N4 donor macrocyclic Schiff base ligands and their Ru (II), Pd (II), Pt (II) metal complexes for biological studies and catalytic oxidation of didanosine in pharmaceuticals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 97, 189–196 (2012)
N.P. Ebosie, M.O. Ogwuegbu, G.O. Onyedika, F.C. Onwumere, Biological and analytical applications of Schiff base metal complexes derived from salicylidene-4-aminoantipyrine and its derivatives: a review. J. Iran. Chem. Soc. (2021). https://doi.org/10.1007/s13738-021-02265-1
M. Ghosh, M. Layek, M. Fleck, R. Saha, D. Bandyopadhyay, Synthesis, crystal structure, and antibacterial activities of mixed ligand copper(II) and cobalt(II) complexes of a NNS Schiff base. Polyhedron 85, 312–319 (2015)
T.H. Sanatkar, A. Khorshidi, E. Sohouli, J. Janczak, Synthesis, crystal structure, and characterization of two Cu(II) and Ni(II) complexes of a tetradentate N2O2 Schiff base ligand and their application in fabrication of a hydrazine electrochemical sensor. Inorganica Chim. Acta. 506, 119537 (2020)
S. Kumari, D. Yadav, S.K. Sharma, Cu(II) Schiff base complex grafted guar gum: catalyst for benzophenone derivatives synthesis. Appl. Catal. A-Gen. 601, 117529 (2020)
A.A. Soliman, G.G. Mohamed, Study of the ternary complexes of copper with salicylidene-2-aminothiophenol and some amino acids in the solid-state. Thermochim. Acta. 421, 151–159 (2004)
N. Zare, A. Zabardasti, A. Mohammadi, F. Azarbani, Synthesis of spherical Fe2O3 nanoparticles from the thermal decomposition of iron (III) nano-structure complex: DFT studies and evaluation of the biological activity. Bioorg. Chem. 80, 334–346 (2018)
A. Khan, T. Al-Bahri, A. Al-Haddad, Adsorption of phenol based organic pollutants on activated carbon from multi-component dilute aqueous solutions. Water Res. 31, 2102–2112 (1997)
N. Hosoya, K. Motomura, E. Tagawa, M. Nagano, C. Ogiwara, H. Hosoya, Effects of the fungicide ortho-phenylphenol (OPP) on the early development of sea urchin eggs. Mar. Environ. Res. 143, 24–29 (2019)
F.C. Macintosh, The toxicity of diphenyl and o-phenyl-phenol. Analyst. 70, 334–335 (1945)
H. Karimi-Maleh, C.T. Fakude, N. Mabuba, G.M. Peleyeju, O.A. Arotiba, The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 554, 603–610 (2019)
W. Shen, Y. Mu, B. Wang, Z. Ai, L. Zhang, Enhanced aerobic degradation of 4-chlorophenol with iron-nickel nanoparticles. Appl. Surf. Sci. 393, 316–324 (2017)
M. Kazemipour, M. Ansari, A. Mohammadi, H. Beitollahi, R. Ahmadi, Use of adsorptive square-wave anodic stripping voltammetry at carbon paste electrode for the determination of amlodipine besylate in pharmaceutical preparations. J. Anal. Chem. 64, 65–70 (2009)
R. Karthik, R. Sasikumar, S.M. Chen, J.V. Kumar, A. Elangovan, V. Muthuraj, M.S. Elshikh, A highly sensitive and selective electrochemical determination of non-steroidal prostate anti-cancer drug nilutamide based on f-MWCNT in tablet and human blood serum sample. J. Colloid Interface Sci. 487, 289–296 (2017)
H. Mahmoudi Moghaddam, H. Beitollahi, S. Tajik, M. Malakootian, H. Karimi Maleh, Simultaneous determination of hydroxylamine, and phenol using a nanostructure-based electrochemical sensor. Environ. Monit. Assess. 186, 7431–7441 (2014)
T.W. Chen, A.S. Vasantha, S.M. Chen, D.A. Al Farraj, M.S. Elshikh, R.M. Alkufeidy, M.M. Al Khulaifi, Sonochemical synthesis and fabrication of honeycomb like zirconium dioxide with chitosan modified electrode for sensitive electrochemical determination of anti-tuberculosis (TB) drug. Ultrasonics Sonochem. 59, 104718 (2019)
S. Tajik, H. Beitollahi, A sensitive chlorpromazine voltammetric sensor based on graphene oxide modified glassy carbon electrode. Anal. Bioanal. Chem. Res. 6, 171–182 (2019)
Y. Wu, Y. Wu, X. Lv, W. Lei, Y. Ding, C. Chen, Q. Hao, A sensitive sensing platform for acetaminophen based on palladium and multi-walled carbon nanotube composites and electrochemical detection mechanism. Mater. Chem. Phys. 239, 121977 (2020)
H. Beitollahi, F. Movahedifar, S. Tajik, S. Jahani, A review on the effects of introducing CNTs in the modification process of electrochemical sensors. Electroanalysis 31, 1195–1200 (2019)
C. Raril, J.G. Manjunatha, A simple approach for the electrochemical determination of vanillin at ionic surfactant modified graphene paste electrode. Microchem. J. 154, 104575 (2020)
H. Mahmoudi-Moghaddam, S. Tajik, H. Beitollahi, Highly sensitive electrochemical sensor based on La3+-doped Co3O4 nanocubes for determination of sudan I content in food samples. Food Chem. 286, 191–196 (2019)
T.W. Chen, U. Rajaji, S.M. Chen, S. Chinnapaiyan, R.J. Ramalingam, Facile synthesis of mesoporous WS2 nanorods decorated N-doped RGO network modified electrode as portable electrochemical sensing platform for sensitive detection of toxic antibiotic in biological and pharmaceutical samples. Ultrasonics Sonochem. 56, 430–436 (2019)
M.A. Khalilzadeh, S. Tajik, H. Beitollahi, R.A. Venditti, Green synthesis of magnetic nanocomposite with iron oxide deposited on cellulose nanocrystals with copper (Fe3O4@CNC/Cu): investigation of catalytic activity for the development of a venlafaxine electrochemical sensor. Ind. Eng. Chem. Res. 59, 4219–4228 (2020)
A.S. Castro, M.M.T. de Menezes, G.M. Alves, M.F. de Oliveira, Voltammetric analysis of cocaine hydrochloride at carbon paste electrode chemically modified with N, N’-ethylene-bis-(salicylideneiminato) manganese(II) Schiff base complex. Microchem. J. 153, 104399 (2020)
H. Beitollahi, M.A. Khalilzadeh, S. Tajik, M. Safaei, K. Zhang, H.W. Jang, M. Shokouhimehr, Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 5, 2049–2059 (2020)
T.W. Chen, U. Rajaji, S.M. Chen, A. Muthumariyappan, M.M. Al Mogren, R.J. Ramalingam, M. Hochlaf, Facile synthesis of copper(II) oxide nanospheres covered on functionalized multiwalled carbon nanotubes modified electrode as a rapid electrochemical sensing platform for super-sensitive detection of antibiotic. Ultrasonics Sonochem. 58, 104596 (2019)
M.R. Ganjali, H. Salimi, S. Tajik, H. Beitollahi, M. Rezapour, B. Larijani, Application of Fe3O4@ SiO2/MWCNT film on glassy carbon electrode for the sensitive electroanalysis of levodopa. Int. J. Electrochem. Sci. 12, 5243–5253 (2017)
H. Beitollahi, Z. Dourandish, S. Tajik, M.R. Ganjali, P. Norouzi, F. Faridbod, Application of graphite screen printed electrode modified with dysprosium tungstate nanoparticles in voltammetric determination of epinephrine in the presence of acetylcholine. J. Rare Earths 36, 750–757 (2018)
G.G. Gerent, A. Spinelli, Magnetite-platinum nanoparticles-modified glassy carbon electrode as electrochemical detector for nitrophenol isomers. J. Hazard. Mater. 330, 105–115 (2017)
M.R. Aflatoonian, S. Tajik, B. Mohtat, B. Aflatoonian, I. Sheikh Shoaie, H. Beitollahi, K. Zhang, H.W. Jang, M. Shokouhimehr, Direct electrochemical detection of clozapine by RuO 2 nanoparticles-modified screen-printed electrode. RSC Adv. 10, 13021–13028 (2020)
G. Congur, A. Erdem, PAMAM dendrimer modified screen printed electrodes for impedimetric detection of miRNA-34a. Microchem. J. 148, 748–758 (2019)
H. Xie, X. Li, G. Luo, Y. Niu, R. Zou, C. Yin, G. Li, Nano-diamond modified electrode for the investigation on direct electrochemistry and electrocatalytic behavior of myoglobin. Diam. Relat. Mater. 97, 107453–107461 (2019)
S. Kannan, J.I. Son, J.E. Yang, Y.B. Shim, Electrochemical polymerization of ruthenium(II) complex and application to acetaminophen analysis. Bull. Korea. Chem. Soc. 32, 1341–1345 (2011)
B. Dinesh, R. Saraswathi, Electrochemical synthesis of nanostructured copper-curcumin complex and its electrocatalytic application towards reduction of 4-nitrophenol. Sensor. Actuat. B-Chem. 253, 502–512 (2017)
H. Beitollahi, S. Nekooei, Application of a modified CuO nanoparticles carbon paste electrode for simultaneous determination of isoperenaline, acetaminophen and N-acetyl-L-cysteine. Electroanalysis 28, 645–653 (2016)
M. Shahbakhsh, M. Noroozifar, Copper polydopamine complex/multiwalled carbon nanotubes as novel modifier for simultaneous electrochemical determination of ascorbic acid, dopamine, acetaminophen, nitrite and xanthine. J. Solid. State. Electrochem. 22, 3049–3057 (2018)
F. Arduini, C. Zanardi, S. Cinti, F. Terzi, D. Moscone, G. Palleschi, R. Seeber, Effective electrochemical sensor based on screen-printed electrodes modified with a carbon black-Au nanoparticles composite. Sens. Actuators B Chem. 212, 536–543 (2015)
K.F. Chan, H.N. Lim, N. Shams, S. Jayabal, A. Pandikumar, N.M. Huang, Fabrication of graphene/gold-modified screen-printed electrode for detection of carcinoembryonic antigen. Mater. Sci. Eng. C 58, 666–674 (2016)
C.W. Foster, J.P. Metters, D.K. Kampouris, C.E. Banks, Ultraflexible screen printed graphitic electroanalytical sensing platforms. Electroanalysis 26, 262–274 (2014)
P. Bollella, G. Fusco, D. Stevar, L. Gorton, R. Ludwig, S. Ma, H. Boer, A. Koivula, C. Tortolini, G. Favero, R. Antiochia, A glucose/oxygen enzymatic fuel cell based on gold nanoparticles modified graphene screen-printed electrode. Proof-of-concept in human saliva. Sens. Actuators B Chem. 256, 921–930 (2018)
A. Wong, A. MartinSantos, E. LuizFava, O. Fatibello-Filho, M.D.P. Taboada Sotomayor, Voltammetric determination of 17β-estradiol in different matrices using a screen-printed sensor modified with CuPc, Printex 6L carbon and Nafion film. Microchem. J. 147, 365–373 (2019)
E. Tozzo, S. Romera, M.P. dos Santos, M. Muraro, H.D.A. Regina, L.M. Liao, E.R. Dockal, Synthesis, spectral studies and X-ray crystal structure of N, N′-(±)-trans-1, 2-cyclohexylenebis (3-ethoxysalicylideneamine) H2 (t-3-EtOsalchxn). J. Mol. Struct. 876, 110–120 (2008)
O. Fatibello-Filho, E.R. Dockal, L.H. Marcolino-Junior, M.F. Teixeira, Electrochemical modified electrodes based on metal-salen complexes. Anal. Lett. 40, 1825–1852 (2007)
A. Panda, S. Panda, K. Srivastava, H.B. Singh, Chemistry of selenium/tellurium-containing Schiff base macrocycles. Inorg. Chim. Acta. 372, 17–31 (2011)
W. Al Zoubi, N. Al Mohanna, Membrane sensors based on Schiff bases as chelating ionophores—a review. Spectrochimica Acta Part A Mol. Biomol. 132, 854–870 (2014)
A. Ourari, B. Ketfi, S.I.R. Malha, A. Amine, Electrocatalytic reduction of nitrite and bromate and their highly sensitive determination on carbon paste electrode modified with new copper Schiff base complex. J. Electroanal. Chem. 797, 31–36 (2017)
Rigaku Oxford Diffraction, CrysAlisPro Software System (Rigaku Corporation, Oxford, 2020)
L. Palatinus, G. Chapuis, SUPERFLIP–a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 40, 786–790 (2007)
V. Petricek, M. Dusek, L. Palatinus, Crystallographic computing system JANA2006: general features. Z. Kristallog. Cryst. Mater. 229, 345–352 (2014)
J. Rohlicek, M. Husak, a new version of a program for fast interactive visualization of electron and similar density maps optimized for small molecules. J. Appl. Cryst. 40, 600–601 (2005)
H. Raouf, S.A. Beyramabadi, S. AllamehA, Morsali, Synthesis, experimental and theoretical characterizations of a 1, 2, 4-triazole Schiff base and its nickel(II) complex. J. Mol. Struct. 1179, 779–786 (2019)
I. Yilmaz, H. Temel, H. Alp, Synthesis, electrochemistry and in situ spectroelectrochemistry of a new Co (III) thio Schiff-base complex with N, N′-bis (2-aminothiophenol)-1, 4-bis (carboxylidenephenoxy) butane. Polyhedron 27, 125–132 (2008)
L.H. Abdel-Rahman, A.M. Abu-Dief, R.M. El-Khatib, S.M. Abdel-Fatah, Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg. Chem. 69, 140–152 (2016)
H. Kargar, V. Torabi, A. Akbari, R. Behjatmanesh-Ardakani, M.N. Tahir, Synthesis, characterization, crystal structure and DFT studies of a palladium(II) complex with an asymmetric Schiff base ligand. J. Mol. Struct. 1179, 732–738 (2019)
A.A.A. Aziz, F.M. Elantabli, H. Moustafa, S.M. El-Medani, Spectroscopic, DNA binding ability, biological activity, DFT calculations and non linear optical properties (NLO) of novel Co(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes with ONS Schiff base. J. Mol. Struct. 1141, 563–576 (2017)
A.A. Soliman, G.G. Mohamed, Study of the ternary complexes of copper with salicylidene-2-aminothiophenol and some amino acids in the solid state. Thermochim. Acta 421, 151–159 (2004)
L.H. Abdel-Rahman, A.M. Abu-Dief, R.M. El-Khatib, S.M. Abdel-Fatah, Sonochemical synthesis, DNA binding, antimicrobial evaluation and in vitro anticancer activity of three new nano-sized Cu(II), Co(II) and Ni(II) chelates based on tri-dentate NOO imine ligands as precursors for metal oxides. J. Photochem. Photobiol B Biol. 162, 298–308 (2016)
R.H. Taha, Z.A. El-Shafiey, A.A. Salman, E.M. El-Fakharany, M.M. Mansour, Synthesis and characterization of newly synthesized Schiff base ligand and its metal complexes as potent anticancer. J. Mol. Struct. 1181, 536–545 (2019)
Z. Abbasi, M. Salehi, A. Khaleghian, M. Kubicki, In vitro cytotoxic activity of a novel Schiff base ligand derived from 2-hydroxy-1-naphthaldehyde and its mononuclear metal complexes. J. Mol. Struct. 1173, 213–220 (2018)
M. Pervaiz, I. Ahmad, M. Yousaf, S. Kirn, A. Munawar, Z. Saeed, A. Rashid, Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochimica Acta Part A Mol. Biomol Spectr. 206, 642–649 (2019)
S. Alyar, C. Sen, H. Alyar, S. Adem, A. Kalkanci, U.O. Ozdemir, Synthesis, characterization, antimicrobial activity, carbonic anhydrase enzyme inhibitor effects, and computational studies on new Schiff bases of Sulfa drugs and their Pd(II), Cu(II) complexes. J. Mol. Struct. 1171, 214–222 (2018)
M. Biabani, H. Saravani, V. Eigner, M. Dusek, A novel coordination polymer of Cd(II) based on 2-mercaptopyrimidine: sonochemical synthesis, characterization, and antibacterial properties. J. Mol. Struct. 1166, 470–478 (2018)
H. Sadeghzadeh, A. Morsali, Sonochemical synthesis and characterization of nano-belt lead(II) coordination polymer: new precursor to produce pure phase nano-sized lead(II) oxide. Ultrasonics Sonochem. 18, 80–84 (2011)
M. Saif, H.F. El-Shafiy, M.M. Mashaly, M.F. Eid, A.I. Nabeel, R. Fouad, Synthesis, characterization, and antioxidant/cytotoxic activity of new chromone Schiff base nano-complexes of Zn(II), Cu(II), Ni(II) and Co(II). J. Mol. Struct. 1118, 75–82 (2016)
D.D. Bui, J. Hu, P. Stroeven, Particle size effect on the strength of rice husk ash blended gap-graded Portland cement concrete. Cem Concr Compos. 27, 357–366 (2005)
H. Karimi-Maleh, C.T. Fakude, N. Mabuba, G.M. Peleyeju, A. Arotiba, The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 554, 603–610 (2019)
D. Tishkevich, S. Grabchikov, T. Zubar, D. Vasin, S. Trukhanov, A. Vorobjova, D. Yakimchuk, A. Kozlovskiy, M. Zdorovets, S. Giniyatova, D. Shimanovich, Early-stage growth mechanism and synthesis conditions-dependent morphology of nanocrystalline Bi films electrodeposited from perchlorate electrolyte. Nanomater. 10, 1245 (2020)
A.J. Bard, L.R. Faulkner, Fundamentals and applications, 2nd edn, p. 864
Acknowledgements
The authors thank the University of Sistan and Baluchestan and the Graduate University of Advanced Technology, Kerman, Iran for the support of this research work. The crystallographic part was supported by the project 18-10504S of the Czech Science Foundation using the CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fazli, M., Akbarzadeh-T, N., Beitollahi, H. et al. New Schiff base ligand N-(2-hydroxy-1-naphthylidene)-2-methyl aniline and its nano-sized copper(II) complex: synthesis, characterization, crystal structure and application as an electrochemical sensor of 2-phenylphenol in the presence of 4-chlorophenol. J Mater Sci: Mater Electron 32, 25118–25136 (2021). https://doi.org/10.1007/s10854-021-06967-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06967-3