Abstract
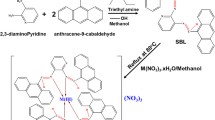
A novel fluorescent ligand (H2LCl⋅1.5CH3OH, 1) was synthesized and metal complexes of 1 with Mn(II), Fe(III), Ni(II), Cu(II), and Zn(II) were obtained as Mn(HL)2Cl2 (2), Fe(HL)2Cl3⋅3H2O (3), Ni(L)(HL)Cl⋅8H2O (4), Cu(HL)Cl2⋅4H2O (5), Zn(H2L)Cl3 (6), respectively. These compounds were identified by spectroscopic methods, elemental analysis, molar conductivity, and single-crystal X-ray crystallography. According to the crystal structure of 4 nickel (II), center is surrounded by two ligands in a distorted octahedral geometry. The ligand and its complexes are soluble in water and have excellent stability. In vitro anti-proliferative activity of these compounds was evaluated against human breast adenocarcinoma (MCF-7) and human lipo-sarcoma (SW-872) as cancer cells and human fibroblasts (HFF-2) as normal cells by MTT assay. Interestingly, complex 5 exhibited excellent activity against both cancer cells with low IC50 value 22.18 ± 0.35 μg/mL (35.66 ± 0.56 μM) for SW-872 and 79.41 ± 3.54 μg/mL (127.6 ± 5.69 μM) for MCF-7 among the compounds and in comparison with paclitaxel (PTX) which acts finely. Morphological changes were evaluated by flow cytometry that revealed apoptosis is the main cause of cell death. Likewise, cell cycle studies indicated the cell cycle arrest in the G1 and S phases for complex 5 against MCF-7 and SW-872 cancer cells, while complex 6 could arrest the MCF-7 and SW-872 cells in G2 and G1 phases, respectively. All of the compounds are fluorescent which enabled us to monitor the uptake and intracellular distribution in living human cancer cells by fluorescence microscopy.
Graphical abstract














Similar content being viewed by others
Data availability
Data will be made available upon reasonable request.
References
Foo JB, Shan Ng L, Lim JH, Tan PX, Lor YZ, Loo JSE, Low ML, Chan LC, Beh CY, Leong SW, Yazan LS, Tor YS, Howa CW (2019) RSC Adv 9:18359–18370
Elamathi C, Fronczek FR, Madankumar A, Prabhakaran R (2020) New J Chem 44:4158–4170
Al-Harbi RAK, El-Sharief MAMSh, Abbas SY (2019) Bioorg Chem 90:103088–103097
Fang Y, Li J, Han P, Han Q, Li M (2018) Toxicol Res 7:987–993
Kalaiarasi G, Dharani S, Lynch VM, Prabhakarana R (2019) Dalton Trans 48:12496–12511
Dankhoff K, Gold M, Kober L, Schmitt F, Pfeir L, Dürrmann A, Kostrhunova H, Rothemund M, Brabec V, Schobert R, Weber B (2019) Dalton Trans 48:15220–15230
Jin J, Hu J, Qin Y, Zhang J, Zhao J, Yueb L, Hou H (2019) New J Chem 43:19286–19297
Cueva-Alique IDL, Muñoz-Moreno L, Torre-Rubio EDL, Bajo AM, Gude L, Cuenca T, Royo E (2019) Dalton Trans 48:14279–14293
Lin X, Liu Y, Xie C, Bao W, Shen J, Xu J (2017) RSC Adv 7:26478–26486
Shanmugapriya A, Dallemer F, Prabhakaran R (2018) New J Chem 42:18850–18864
Cavalcante CDQO, Arcanjo DDS, Silva GGD, Oliveira DMD, Gatto CC (2019) New J Chem 43:11209–11221
Zampakou M, Hatzidimitriou AG, Papadopoulos AN, Psomas G (2015) J Coord Chem 68(24):4355–4372
Kalaiarasi G, Jain R, Puschman H, Chandrika SP, Preethi K, Prabhakaran R (2017) New J Chem 41:2543–2560
Zampakou M, Balala S, Perdih F, Kalogiannis S, Turel I, Psomas G (2015) RSC Adv 5:11861–11872
Balakrishnan N, Haribabu J, Dhanabalan AK, Swaminathan S, Sun S, Dibwe DF, Bhuvanesh N, Awale S, Karvembu R (2020) Dalton Trans 49:9411–9424
Kathiresan S, Mugesh S, Annaraj J, Murugan M (2017) New J Chem 41:1267–1283
Singh NK, Yadav PN, Kumbhar AA, Pokhrel YR (2020) J Inorg Biochem 210:111134
Sarkar S, Mondal T, Roy S, Saha R, Ghosh AK, Panja SS (2018) New J Chem 42:15157–15169
Adak P, Ghosh B, Bauza A, Frontera A, Herron SR, Chattopadhyay SK (2020) RSC Adv 10:12735–12746
Wang Y, Fang Y, Zhao M, Li M, Ji Y, Han Q (2017) Med Chem Commun 8:2125–2132
Matesanz AI, Jimenez-Faraco E, Ruiz MC, Balsa LM, Navarro-Ranninger C, Leon IE, Quiroga AG (2018) Inorg Chem Front 5:73–83
Kalaiarasi G, Rajkumar SRJ, Dharani S, Małecki JG, Prabhakaran R (2018) RSC Adv 8:1539–1561
Sirbu A, Palamarciuc O, Babak MV, Lim JM, Ohui K, Enyedy EA, Shova S, Darvasiova D, Rapta P, Ang WH, Arion VB (2017) Dalton Trans 46:3833–3847
Milunovic MNM, Enyedy EA, Nagy NV, Kiss T, Trondl R, Jakupec MA, Keppler BK, Krachler R, Novitchi G, Vladimir B (2012) Arion VB. Inorg Chem 51:9309–9321
Milunovic MNM, Dobrova A, Novitchi G, Gligorijevic N, Radulovic S, Kozisek J, Rapta P, Enyedy EA, Arion VB (2017) Eur J Inorg Chem 40:4773–4783
Bacher F, Enyedy EA, Nagy NV, Rockenbauer A, Bognar GM, Trondl R, Novak MS, Klapproth E, Kiss T, Arion VB (2013) Inorg Chem 52:8895–8908
Milunovic MNM, Palamarciuc O, Sirbu A, Shova A, Dumitrescu D, Dvoranova D, Rapta P, Petrasheuskaya TV, Enyedy EA, Spengler G, Ilic M, Sitte HH, Lubec G, Arion VB (2020) Biomolecules 10:1213
Cowley AR, Davis J, Dilworth JR, Donnelly PS, Dobson R, Nightingale A, Peach JM, Shore B, Kerr D, Seymour L (2005) Chem Commun 845–847
Kowol CR, Trondl R, Arion VB, Jakupec MA, Lichtscheidl I, Keppler BK (2010) Dalton Trans 39:704–706
Hickey JL, James JL, Henderson CA, Price KA, Mot AI, Buncic G, Crouch PJ, White JM, White AR, Smith TA, Donnelly PS (2015) Inorg Chem 54:9556–9567
Mirzaahmadi A, Hosseini-Yazdi SA, Safarzadeh E, Baradaran B, Samolova E, Dusek M (2019) J Mol Liq 293:111412–111422
Mirzaahmadi A, Hosseini-Yazdi SA, Mahdavi M, Samolova E, Dusek M (2021) Polyhedron 202:115205
Berkebile JM, Fries AH (1948) J Chem Educ 25:617–618
Cymerman-Craig J, Moyle M, White RA (1956) Org Synth 36:56–57
Li Z, Xiang Y, Tong A (2008) Anal Chim Acta 619:75–80
Basu A, Thiyagarajan D, Kar C, Ramesh A, Das G (2013) RSC Adv 3:14088–14098
Samanta S, Manna U, Ray T, Das G (2015) Dalton Trans 44:18902–18910
Neamtu M, Macaev F, Boldescu V, Hodoroaba VD, Nadejde C, Schneider RJ, Paul A, Ababei G, Panne U (2016) Appl Catal B Environ 183:335–342
Evans DF (1959) J Chem Soc 2003–2005
Basu A, Das G (2011) Dalton Trans 40:2837–2843
Kaushal M, Lobana TS, Nim L, Kaur J, Bala R, Hundal G, Arora DS, Garcia-Santos I, Duff CE, Jasinski JP (2018) New J Chem 42:15879–15894
Zhang Z, Yu P, Gou Y, Zhang J, Li S, Cai M, Sun H, Yang F, Liang H (2019) J Med Chem 62:10630–10644
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edition. Amsterdam
Mahendiran D, Amuthakala S, Bhuvanesh NSP, Kumarc RS, Rahiman AK (2018) RSC Adv 8:16973–16990
Qi J, Liu T, Zhao W, Zheng X, Wang Y (2020) RSC Adv 10:18553–18559
Bharathi S, Mahendiran D, Kumar RS, Kim YG, Gajendiran M, Kim K, Rahiman AK (2019) Chem Res Toxicol 32:1554–1571
Acknowledgements
We thank the University of Tabriz-Iran and Iran National Science Foundation (INSF) for funding aspect of this present research. Also, we would like to thank Ardabil University of Medical Sciences and Immunology Research Center of Tabriz University of Medical Sciences. The authors kindly thank the project SOLID21 CZ.02.1.01/0.0/0.0/16_019/0000760 from Operational Programme Research, Development and Education financed by European Structural and Investment Funds and the Czech Ministry of Education, Youth and Sports for supporting the crystallographic part of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
775_2023_2001_MOESM1_ESM.pdf
Supplementary file1Supplementary data associated 1H, 13C NMR of 1 and 6 and TGA-DTA diagrams for 2–5. Also, X-ray crystallography section was included. (PDF 4518 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feizpour, S., Hosseini-Yazdi, S.A., Safarzadeh, E. et al. A novel water-soluble thiosemicarbazone Schiff base ligand and its complexes as potential anticancer agents and cellular fluorescence imaging. J Biol Inorg Chem 28, 457–472 (2023). https://doi.org/10.1007/s00775-023-02001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-023-02001-5