Abstract
Background
The volume effect of fat grafting is highly dependent on the presence of viable adipocytes and other nucleated cells within the lipoaspirate. We suspected that one of the crucial factors influencing cell viability is the negative pressure applied during the fat graft harvesting and the suitability of various harvest sites when compared to others. Despite much discussion, there is no consensus on the optimal negative pressure or the best site for harvesting so we designed an experiment to test this.
Methods
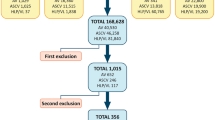
Fat graft taken under low negative pressure (− 200 mmHg) or high negative pressure (− 700 mmHg) from the thigh or abdominal regions from 21 healthy human donors was evaluated. The principal variables studied were: a) total number and viability of nucleated cells, b) liposuction duration and c) blood admixture. Other variables studied were body mass index, the impact of age and enzymatic digestion.
Results
The absolute number and viability of nucleated cells and the blood admixture did not differ significantly between lipoaspirates obtained under different vacuum conditions or from different regions. The time taken to acquire the same volume of lipoaspirate was significantly increased using low negative pressure. The time taken to collect cells in the thigh region significantly increased with increasing BMI but this correlation was not found when harvesting in the abdominal region. The BMI and age did not impact the results in any of the measured variables. The enzymatic digestion rate was independent of the negative pressure used to harvest.
Conclusion
Our results indicate that neither the negative pressure used nor the area chosen has any significant influence on the viability and yield of harvested cells. The time taken to obtain lipoaspirate using low pressure is significantly longer than when using high pressure. No significant difference was found in the value of blood admixture using different vacuum pressures, and no correlation exists between the body mass index and the cell viability or age of the patients and the time of liposuction.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine Ratings, please refer to Table of Contents or online Instructions to Authors www.springer.com/00266.











Similar content being viewed by others
References
Coleman SR (2001) Structural fat grafts: the ideal filler? Clin Plast Surg 28:111–119
Coleman SR (2002) Hand rejuvenation with structural fat grafting. Plast Reconstr Surg. 110:1731–1744
Coleman SR (1995) Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 19:421–425
Coleman SR (1997) Facial recontouring with lipostructure. Clin Plast Surg 24:347–367
Bank J, Fuller SM, Henry GI, Zachary LS (2014) Fat grafting to the hand in patients with Raynaud phenomenon. Plast Reconstr Surg 133(5):1109–1118
Kakagia D, Pallua N (2014) Autologous fat grafting: in search of the optimal technique. Surg Innov 21:327–336
Khouri RK Jr, Khouri RK (2017) Current clinical applications of fat grafting. Plast Reconstr Surg 140(3):466e–486e
Tuncel U, Kurt A, Gumus M, Ayodogdu O, Guzei N, Demir O (2017) Preliminary results with non-centrifuged autologous fat graft and percutaneous aponeurotomy for treating Dupuytren’s disease. Hand Surg Rehabil 36(5):350–354
Condé-Green A, Gontijo de Amorim NF, Pitanguy I (2010) Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. J Plast Reconstr Aesthet Surg 63:1375–1381
Peer LA (1950) Loss of weight and volume in human fat graft, with postulation of a cell survival theory. Plast Reconstr Surg 5:217–221
Peer LA (1955) Cell survival theory versus replacement theory. Plast Reconstr Surg 16:161–168
Tremolada C, Palmieri G, Ricordi C (2010) Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplant 19:1217–1223
Gutowski KA (2009) ASPS fat graft task force. current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg 124:272–280
Salinas HM, Broelsch GF, Fernandes JR, McCormac MC, Meppelink AM, Randolph MA, ColwellAS AWG Jr (2014) Comparative analysis of processing methods in fat grafting. Plast Reconstr Surg 134:675–683
Gabriel A, Champaneria MC, Maxwell GP (2015) Fat grafting and breast reconstruction: tips for ensuring predictability. Gland Surg 4(3):232–243
Ince B, Oltulu P, Yildirim MEC, Ismayilzade M, Dadaci M (2019) Effects of aspiration time on immediate viability of adipocyte cell in ultrasound-assisted liposuction (UAL) and in traditional suction-assisted lipectomy (SAL). J Plast Surg Hand Surg 53(1):14–19
Özkaya O, Egemen O, Barutca SA, Akan M (2013) Long-term clinical outcomes of fat grafting by low-pressure aspiration and slow centrifugation (Lopasce technique) for different indications. J Plast Surg Hand Surg 47(5):394–398
Alharbi Z, Opländer C, Almakadi S, Fritz A, Vogt M, Pallua N (2013) Conventional vs. micro-fat harvesting: How fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J Plast Reconstr Aesthetic Surg 66:1271–1278
Von Heimburg D, Hemmrich K, Haydarlioglu S, Staiger H, Pallua N (2004) Comparison of viable cell yield from excised versus aspirated adipose tissue. Cells Tissues Organs 178(2):87–92
Fraser J, Wulur I, Alfonso Z, Zhu M, Wheeler E (2007) Differences in stem and progenitor cell yield in different subcutaneous adipose tissue depots. Cytotherapy 9(5):459–467
Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG (2013) The effect of pressure and shear on autologous fat grafting. Plast Reconstr Surg 131:1125–1136
Nguyen A, Pasyk KA, Bouvier TN, Hasset CA, Argenta LC (1990) Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg 85(3):378–386
Kononas TC, Bucky LP, Hurley C, May JW (1993) The fate of suctioned and surgically removed fat after reimplantation for soft-tissue augmentation: a volumetric and histologic study in the rabbit. Plast Reconstr Surg 91(5):763–768
Gonzalez AM, Lobocki C, Kelly CP, Jackson IT (2007) An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg 120(1):285–294
Noaves F, dos Reis N, Baroudi R (1998) Counting of live fat cells used in lipoinjection procedures. Aesth Plast Surg 22:12–15
Shiffman MA, Mirrafati S (2001) Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg 27:819–826
Pu LLQ, Cui X, Fink BF, Cibull ML, Gao D (2005) The viability of fatty tissues within adipose aspirates after conventional liposuction a comprehensive study. Annals Plast Surg 54(3):288–292
Smith P, Adams WP, Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA (2006) Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Recostr Surg 117(6):1836–1844
Tambasco D, Arena V, Grussu F, Cervelli D (2013) Adipocyte damage in relation to different pressures generated during manual lipoaspiration with syringe. Plast Reconstr Surg 131(4):645e–646e
Cheriyan T, Kao HK, Qiao X, Guo L (2014) Low harvest pressure enhances autologous fat graft viability. Plast Reconstr Surg 133(6):1365–1368
Leong DT, Hutmacher DW, Chew FT, Lim TC (2005) Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J Dermatol Sci 37:169–176
Mojallal A, Auxenfans C, Lequeux C, Braye F, Damour O (2008) Influence of negative pressure when harvesting adipose tissue on cell yield of the stromal-vascular fraction. Bio Med Mater Eng 18(4–5):193–197
Keck M, Kober J, Riedl O, Kitzninger HB, Wolf S, Stulnig TM, Zayda M, Gugerell A (2014) Power assisted liposuction to obtain adipose-derived stem cells: impact on viability and differentiation to adipocytes in comparison to manual aspiration. J Plast Reconstr Aesthetic Surg 67(1):e1-8
Barzelay A, Levy R, Kohn E, Sella M, Shani N, Meilik B, Entin-Meer M, Gur E, Loewenstein A, Barak A (2015) Power-assisted liposuction versus tissue resection for the isolation of adipose tissue–derived mesenchymal stem cells: phenotype, senescence and multipotency at advanced passages. Aesthet Surg J. 35(7): NP230–NP240
Bony C, Cren M, Domergue S, Toupet K, Jorgensen C, Noel D (2016) Adipose mesenchymal stem cells isolated after manual or water-jet-assisted liposuction display similar properties. Front Immunol. doi:https://doi.org/10.3389/fimmu.2015.00655
Duscher D, Luan A, Rennert RC, Atashroo D, Maan ZN, Brett EA, Whittam AJ, Ho N, Lin M, Hu MS, Walmsley GG, Wenny R, Schmidt M, Schilling AF, Machens HG, Huemer GM, Wan DC, Longaker MT, Guntner GC (2016) Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. J Transl Med 14(1):126–138
Charles-de-Sá L, Gontijo de Amorim NF, Dantas D, Han JV, Amable P, Teixeira MVT, Luiz de Ajauro P, Link W, Borojevich R, Rigotti G (2015) Influence of negative pressure on the viability of adipocytes and mesenchymal stem cell, considering the device method used to harvest fat tissue. Aesthet Surg J 35(3):334–344
Chen YW, Wang JR, Liao X, Li SH, Xiao LL, Cheng B, Xie GH, Song JX, Liu HW (2017) Effect of suction pressures on cell yield and functionality of the adipose-derived stromal vascular fraction. J Plast Reconstr Aesthet Surg.
Travnickova M, Pajorova J, Zarubova J, Krocilova N, Molitor M, Bacakova L (2020) The influence of negative pressure and of the harvesting site on the characteristics of human adipose tissue-derived stromal cells from lipoaspirates. Stem Cells International. doi.org/https://doi.org/10.1155/2020/1016231
ASPS evidence‐based clinical practice guideline methodology, December 2016. https://www.plasticsurgery.org/documents/medical-professionals/quality-resources/ASPS-Evidence%E2%80%90Based-Clinical-Practice-Guideline-Methodology.pdf, accessed 12.3.2021
Burns PB, Rohrich RJ, Chung KC (2011) The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 128(1):305–310
Molitor M, Trávníčková M, Měšťák O, Christodoulou P, Sedlář A, Bačáková L, Lucchina S (2021) The influence of high and low negative pressure liposuction and various harvesting techniques on the viability and function of harvested cells – a systematic review of animal and human studies. Aesthetic Plast Surg. Apr 19. doi:https://doi.org/10.1007/s00266-021-02249-9. Online ahead of print.
Vyas KS, Vascones HC, Morrison S, Mogni B, Linton S, Hockensmith L, Kabir T, Zielins E, Najor A, Bakri K, Mardini S (2020) Fat graft enrichment strategies: a systematic review. Plast Reconsr Surg 145(3):827–841
Cucchiani R, Corrales L (2016) The effects of fat harvesting and preparation, air exposure, obesity and stem cell enrichment on adipocyte viability prior to graft transplantation. Aesth Surg J 36(10):1164–1173
Padoin AV, Braga-Silva J, Martins P, Rezende K, Rezende ARR, Grechi B, Gehlen D, Machado DC (2008) Sources of processed lipoaspirate cells: influence of donor site on cell concentration. Plast Reconstr Surg 122:614–618
Qu Y, Mu D, Wang Q, Li Z, Liu T, Fu S, Luan J (2020) Effect of harvest sites on cryopreserved adipose-derived stem cells and asc-enriched fat grafts. Aesth Plast Surg 44:2286–2296
Rohrich RJ, Sorokin ES, Brown SA (2004) In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 113:391–395
Ullmann Y, Shoshani O, Fodor A, Ramon Y, Carmi N, Eldor L, Gilhar A (2005) Searching for the favourable donor site for fat injection: in vivo study using the nude mice model. Dermatol Surg 31:1304–1307
Li K, Gao J, Zhang Z, Li J, Cha P, Liao Y, Wang G, Lu F (2013) Selection of donor site for fat grafting and cell isolation. Aesthetic Plast Surg 37:153–158
Elam MW, Packer D, Schwab J (1997) Reduced negative pressure liposuction (RNPL) could less be more? Int J Aesth Restor Surg. 5(2):101–104
Jin S, Yang Z, Han X, Li F (2021) Blood impairs viability of fat grafts and adipose stem cells: importance of washing in fat processing. Aesthet Surg J 41(1):86–97
Sirinoglu H, Yesiloglu N, Kaya OT, Ercan F, Filinte GT (2017) Effect of tumescent solution on fat survival. Facial Plast Surg 33(3):339–346
Carpaneda CA (1996) Study of aspirated adipose tissue. Aesth. Plast. Surg 20:399–402
Khouri RK, Rigotti G, Cardoso E, Khouri RK Jr, Biggs TM (2014) Megavolume autologous fat transfer: part I. Theory and principles. Plast Reconstr Surg 133:550–557
Khouri RK, Rigotti G, Cardoso E, Khouri RK Jr, Biggs TM (2014) Megavolume autologous fat transfer: part II. Practice and techniques. Plast Reconstr Surg 133:1369–1377
Funding
This study was supported by the Ministry of Health of the Czech Republic (grant No. NU20-08-00208)—authors 1, 2, 5, 6, (including personal fee)—and by the Ministry of Education, Youth and Sports of the Czech Republic within LQ1604 National Sustainability Program II (Project BIOCEV-FAR)—author 2 (including personal fee)—and by the project “BIOCEV” (CZ.1.05/1.1.00/02.0109) authors 2, 6 (including personal fee).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest concerning the research, authorship and publication of this article. Financial funding of research has not any influence on the results of the research in any form.
Human or Animal Rights
The study was approved by institutional Ethical Committee (Na Bulovce University Hospital, Prague). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Written informed consent was obtained from all human participants included in the study. Informed consent was approved by institutional Ethical Committee Na Bulovce University Hospital, Prague.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Molitor, M., Trávníčková, M., Měšťák, O. et al. The Influence of Low- and High-Negative-Pressure Liposuction and Different Harvesting Sites on the Viability and Yield of Adipocytes and Other Nucleated Cells. Aesth Plast Surg 45, 2952–2970 (2021). https://doi.org/10.1007/s00266-021-02396-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-021-02396-z