Abstract
A repetitive pulsed-power modulator, which employs a magnetic pulse compression circuit with a high-speed thyristor switch, was used to study the effects of the pulse repetition rate of input power on the physical and chemical properties of pulsed discharges in water. Positive high-voltage pulses of 20 kV with repetition rates of up to 1 kHz were used to generate a discharge in water using the point-to-plane electrode geometry. By varying the pulse repetition rate, two distinct modes of the discharge plasma were formed in water. The first mode was characterized by the formation of a corona-like discharge propagating through water in the form of streamer channels. The second mode was formed typically above 500 Hz, when the formation of streamer channels in water was suppressed and all plasmas occurred inside a spheroidal aggregate of very fine gas bubbles surrounding the tip of the high-voltage electrode. The production of hydrogen peroxide, degradation of organic dye Acid Orange 7 (AO7) and inactivation of bacteria Escherichia coli by the discharge in water were studied under different discharge plasma modes in dependence on the pulse repetition rate of input power. The efficiency of both chemical and biocidal processes induced by the plasma in water decreased significantly with pulse repetition rates above 500 Hz.
Export citation and abstract BibTeX RIS
1. Introduction
Electrical discharges generated directly in water have recently attracted significant interest for their potential applications in chemical, environmental and disinfection processes such as wastewater treatment, polymer surface modifications or synthesis of nanoparticles [1–4]. A non-thermal plasma produced by these discharges interacts with water molecules to initiate physical and chemical processes in water such as a strong electric field, intense UV radiation, shockwaves and formation of various chemically active species (OH·, H·, O·,
 , H2O2, H2, O2, etc) involving excitation, dissociation and ionization of water in the discharge plasma region [5–7]. Many factors influence the generation and properties of the plasma in water as well as the physical and chemical processes induced by the plasma including mainly input power parameters, liquid-phase properties such as hydrostatic pressure, temperature, solution conductivity, de-aeration (pre-existence of micro-bubbles) of water, and electrode conditions such as electrode material, surface asperities and interfacial properties [8, 9]. The roles of many of these factors are not known explicitly.
, H2O2, H2, O2, etc) involving excitation, dissociation and ionization of water in the discharge plasma region [5–7]. Many factors influence the generation and properties of the plasma in water as well as the physical and chemical processes induced by the plasma including mainly input power parameters, liquid-phase properties such as hydrostatic pressure, temperature, solution conductivity, de-aeration (pre-existence of micro-bubbles) of water, and electrode conditions such as electrode material, surface asperities and interfacial properties [8, 9]. The roles of many of these factors are not known explicitly.
This work investigates the effects of the pulse repetition rate of input power on the properties of pulsed high-voltage discharges in water. Many studies have used ac power sources with frequencies up to 100 kHz, radiofrequency or microwave power to generate plasmas in water [10–16]. In these cases, the major mechanism of initiation of plasmas in water was the breakdown of a water vapour bubble formed by Joule heating at or near the high-voltage electrode, and the power frequency was shown as an important parameter influencing the bubble behaviour and the characteristics of the plasma in water [15–17]. Conversely, the effects of pulse frequency on the properties of pulsed high-voltage discharges in water were rarely studied. Pulsed discharges in water are typically operated at pulse repetition rates of 1–100 Hz, conditions under which a number of studies reporting physical and chemical properties of discharge plasmas exist [2, 6]. Higher frequencies (1–30 kHz) to drive plasmas in water using pulsed power were reported, for example, by Baroch et al [18], who used a bipolar pulse power supply with reduced voltage (<4 kV) to generate pulsed arc discharges in water. The authors did observe the effect of pulse repetition rate on the breakdown voltage of the discharge in water but without further studies of this phenomenon.
Reasons for the limited number of reports on the effects of pulse frequency on the characteristics of plasmas generated in water using pulsed power are related to the high requirements of the pulse power supply generating pulsed discharges in water. Spark-gap switches, the most typically employed, have repetitive performance limited to pulse repetition frequencies of several hundred Hz. Some studies used thyratrons or semi-conductor switches, which are capable of operation at higher repetition rates; however, no effects of pulse frequency on the parameters of pulsed discharges in water were reported [19–21].
This work utilizes a repetitive pulsed-power modulator (PPM), which employs a magnetic pulse compression circuit with a high-speed thyristor switch, for the generation of a pulsed discharge in water [22–24]. The system can generate high-voltage pulses with a repetition rate of up to 1 kHz. We observed that varying the pulse frequency enables us to generate the discharge plasma in water at two distinct modes. The first mode was characterized by the formation of a corona-like discharge propagating through water in the form of streamer channels (hereafter referred to as the streamer mode). The second mode occurred when the pulse repetition rate was increased to above a certain threshold value (typically above 500 Hz), and was characterized by the suppression of the formation of streamer channels in water. Instead, all plasmas occurred inside a spheroidal aggregate of very fine gas bubbles, which formed around the discharge electrode tip very quickly after application of the pulsed high voltage into water (hereafter referred to as the bubble mode). This process significantly changed the environment surrounding the electrode tip from the initial pure liquid phase to a gas–liquid phase in which the discharge formation and its subsequent propagation were considerably different. This work investigates the physical and chemical properties of an underwater discharge produced under these conditions in more detail. The production of hydrogen peroxide, degradation of organic dye Acid Orange 7 (AO7) and inactivation of bacteria Escherichia coli by the discharge in water were measured under different discharge plasma modes to determine the effects of the pulse repetition rate of input power on the plasma chemical and biocidal activity of the pulsed discharge in water. Mechanisms that may contribute to the observed changes in plasma formation and its chemical and biocidal activity with varying pulse frequency are discussed.
2. Experimental
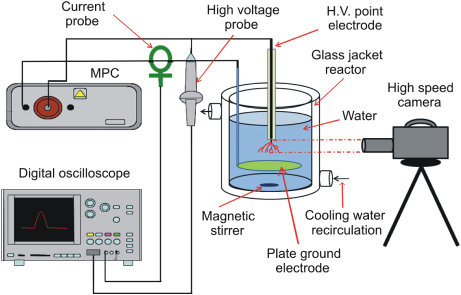
A point-to-plane electrode system immersed in a cylindrical glass reactor (volume 200 ml) equipped with a water cooling jacket was used to generate a pulsed discharge in water (figure 1). The needle high-voltage electrode was made of a sharpened tungsten wire (approximately 0.4 mm in diameter); the grounded electrode was an aluminum plate (diameter 20 mm, thickness 0.2 mm). The tungsten wire was insulated from the surrounding water by ceramic and Teflon tubes, with only the sharpened tip in direct contact with water. The gap distance between the electrodes was 25 mm. The temperature of the aqueous solution inside the reactor was maintained constant by circulation of cooling water (10 °C) through the cooling jacket of the reactor from a controlled temperature water bath (Eyla NCB-1200, Japan). Aqueous solutions used in the experiments were prepared from deionized water by the addition of KCl salt. The initial electrolytic conductivity of these solutions was 100 µS cm−1 in all experiments performed in this work. The volume of liquid used for the experiments was 200 ml. The solutions were mixed by a magnetic bar at the bottom of the glass reactor. Images of discharges in water were captured using a high-speed camera VW-6000 (Keyence, Japan) with a shutter speed of 1/10 000 and a frame rate of 250 fps.
Figure 1. Schematic diagram of the experimental setup used for generation of underwater discharge plasmas.
Download figure:
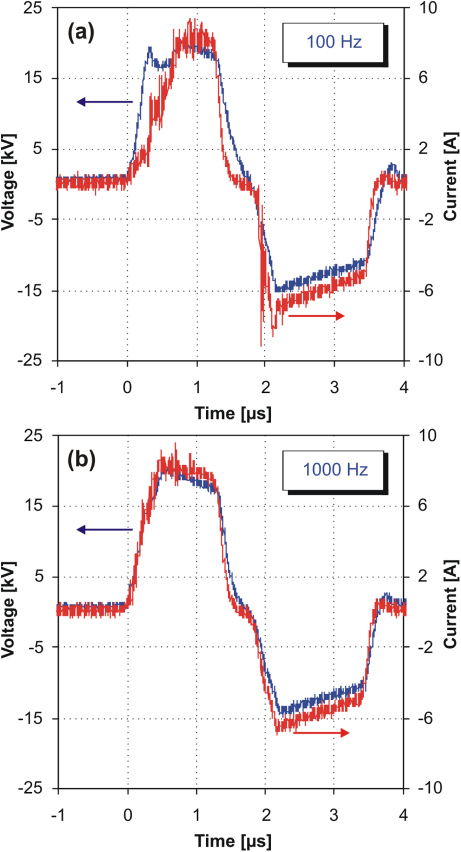
Standard imageA repetitive PPM employing a magnetic pulse compression circuit with a high-speed thyristor switch was used to generate positive pulsed high-voltage discharges in water with a pulse repetition rate of up to 1 kHz, as was used in our previous work [22]. The setup consists of a charger unit (EMI, 500A, TDK-Lambda Americas Inc.), a controller unit (Suematsu Electronic Company, Japan) and a PPM system (developed at the Plasma and Pulsed Power Laboratory, Kumamoto University, Kumamoto, Japan). The experiments were conducted at seven different pulse repetition rates—50, 100, 250, 350, 500, 700 and 1000 Hz—with an output voltage of 20 kV. The experiments were carried out without the use of an output peaking capacitor in the PPM [22]. Electrical measurements were made using a dual-channel Tektronix oscilloscope (AFG3102) with a Tektronix high-voltage probe (P6015A) and a Pearson current probe (model 3972). The discharge pulse energy was determined by the integration of the respective voltage and current waveforms. Figure 2 shows typical waveforms of the output voltage and the current measured for a discharge generated in water (100 µS cm−1) upon application of high-voltage pulses with repetition rates of 100 and 1000 Hz. No significant differences in pulse width, rise time or amplitude of the output voltage were observed except for a slight difference in the shape of the positive half-cycle waveform. The discharge current rise time was faster and pulse width longer for 1000 Hz (450 ns and 1.2 µs full-width at half-maximum (FWHM), respectively) than those for 100 Hz (650 ns and 1.0 µs FWHM, respectively). However, the total pulse discharge energy was very similar in each case (∼250 mJ/pulse). The faster discharge current rise time measured at 1000 Hz was probably caused by the presence of bubbles remaining near the electrode tip from the previous discharge periods, which facilitated electric breakdown in water from the electrode tip.
Figure 2. Voltage and current waveforms of the pulsed discharge in water generated using input power with pulse repetition rates of (a) 100 Hz and (b) 1000 Hz.
Download figure:
Standard imageChemical activity of the discharge was evaluated by measurement of plasma chemical production of H2O2 and degradation of organic dye AO7 (C16H11N2NaO4S, molecular weight: 350.32 g mol−1). H2O2 concentration was determined colourimetrically using the reaction of H2O2 with titanyl ions measuring the absorbance of the peroxotitanium (IV) complex at 410 nm. The dye concentration was determined using absorption spectroscopy (Hitachi U-2900) by measuring the change in absorbance at 484 nm and by scanning the absorption spectra of the dye in the wavelength range from 200 to 700 nm. The initial concentration of the dye solution, prepared from deionized water, was 20 mg l−1. The initial conductivity of the dye solution was adjusted by KCl salt to 100 µS cm−1.
Biocidal effects of the discharge were evaluated on bacteria E. coli. Bacterial suspensions were prepared by preculturing bacteria cells in the Luria-Broth growth medium at 37 °C for 24 h. Bacteria cells were then centrifuged and washed with 0.85% sterilized physiological saline; 4.5 ml of that bacterial suspension was dispersed in 200 ml of NaCl solutions, with a final solution conductivity of 100 µS cm−1. The number of bacteria cells in the liquid suspension was assayed by counting colony forming units (CFUs) cultivated on agar plates. The initial amount of bacteria was 2 × 108 CFU ml−1 in all experiments performed in this study. The viability of the bacteria was determined as the ratio of the concentration of surviving bacteria to the total concentration.
3. Results and discussion
3.1. Effects of pulse frequency on the physical characteristics of the pulsed discharge plasma in water
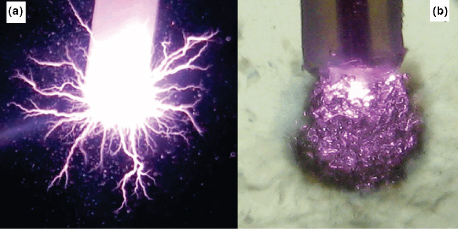
By varying the pulse frequency in the range 1–1000 Hz, the pulsed discharge plasma was generated at two distinct modes in water. Figure 3 shows a typical example of the effect of the pulse repetition rate on the appearance of the pulsed discharge plasma in water generated using high-voltage pulses with repetition rates of 250 and 500 Hz [24]. Apart from the repetition rate, experimental conditions were identical in each case. Figure 3 shows completely different discharge modes due to variation of the pulse repetition rate. The first mode was characterized by the formation of a typical corona-like discharge propagating through water in the form of streamer channels (figure 3(a), referred to as the streamer mode). However, when the pulse repetition rate was increased above a certain threshold value—typically above 500 Hz—the formation of streamer channels in water was suppressed, as shown in figure 3(b) (referred to as the bubble mode). Instead, all plasmas occurred inside a spheroidal aggregate of very fine gas bubbles formed around the discharge electrode tip very soon after the application of high-voltage pulses into water. This process significantly changed the environment surrounding the electrode tip from the initial pure liquid phase to a gas–liquid phase in which the discharge formation and its subsequent propagation were different. Several effects, as discussed below, might contribute to the observed change in plasma formation with varying of pulse frequency.
Figure 3. Images of appearance of the discharge generated in water from the tip of the point electrode using high-voltage pulses with repetition rates of (a) 250 Hz and (b) 500 Hz. © 2009 IEEE. Reprinted, with permission, from [24].
Download figure:
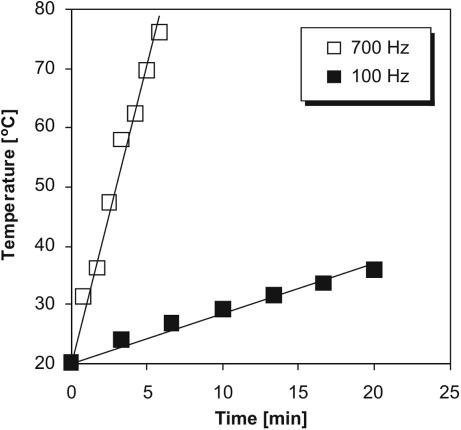
Standard imageWith a higher pulse frequency, thermal processes likely play an important role since input power and contact duration of the plasma with water increased with pulse frequency. Figure 4 shows an increase in bulk temperature of water in the plasma reactor caused by the discharge generated using high-voltage pulses with repetition rates of 100 Hz and 700 Hz (i.e. with power input of 25 W and 175 W, respectively). The same magnitude of applied voltage of 20 kV and a pulse energy of 250 mJ/pulse were used; water in the reactor was not cooled by an external cooling circuit (the total water volume was 200 ml, initial temperature 20 °C, solution conductivity 100 µS cm−1). Figure 4 shows that water was heated up more quickly and to a much higher temperature by the discharge operated at the higher pulse frequency. The temperature of water in the reactor increased by more than 50 °C within 5 min of treatment by the discharge at 700 Hz compared with only 5 °C increase at 100 Hz after the same period.
Figure 4. Variation of bulk temperature of water in the reactor during the discharge plasma treatment using high-voltage pulses with repetition rates of 100 and 700 Hz (volume of water 200 ml, pulse energy 250 mJ, water in the reactor was not cooled by an external cooling circuit).
Download figure:
Standard imageSignificant heat transfer from the discharge into the water surrounding the point electrode tip is also apparent from figure 5(a), which shows a time sequence of shadowgraphs of the region around the electrode tip taken by a high-speed camera during operation of the discharge in water at a pulse repetition rate of 700 Hz. Figure 5(a) indicates large changes in the refractive index of water in the area close to the discharge electrode tip, which were obviously caused by changes in the density/temperature of water. At lower frequencies, such large temperature gradients were not observed. Note that water in the reactor was not cooled by an external cooling circuit in these experiments to visualize processes in water more clearly. Figure 5 also shows that there was rather complex dynamics of bubbles in the discharge region which will be discussed later.
Figure 5. (a) Shadowgraph visualization of temperature changes in water in the region around the tip of the point electrode during operation of the discharge at a pulse repetition rate of 700 Hz. (b) Time evolution of jetting of cluster of small bubbles from the bubble spheroid in the region of point electrode tip during operation of the discharge at a pulse repetition rate of 700 Hz. Water in the reactor was not cooled by an external cooling circuit in these experiments.
Download figure:
Standard imageTo further evaluate the effect of temperature on the formation of the discharge in bubble mode additional tests were conducted using water with an initial temperature of 80 °C and solution conductivity of 100 µS cm−1. In this case, the formation of streamer channels in water was suppressed already with a pulse repetition rate of 350 Hz instead of 500 Hz, which was determined as the threshold value for the formation of bubble mode in water at a temperature of 20 °C and with solution conductivity of 100 µS cm−1. Therefore, it is likely that the effect of pulse frequency on the formation of the discharge in bubble mode was coupled with the local heating of water by the discharge in the area close to the discharge electrode tip. With higher pulse frequency and higher temperature of water the power delivered into the water by the discharge was sufficient to reach a temperature for water evaporation and sustenance of a stable vapour bubble(s) around the discharge electrode tip. The solution conductivity also influenced the behaviour of the discharge in water. In low conductive water (1 µS cm−1), the streamer mode was formed even at a pulse repetition rate of 500 Hz for which otherwise the discharge in bubble mode was observed in more conductive water (>100 µS cm−1) [24].
To some extent, these phenomena are comparable to the features of dc discharges excited in vapour bubbles in water (capillary, diaphragm), in which higher power applied to the liquid advances the thermal effects in water and, thus, the formation of vapour bubble inside the diaphragm (capillary) and its subsequent breakdown [25]. Bruggeman et al [26] observed, for dc-excited discharges generated in water using a capillary electrode, formation of two distinctive discharge modes, which were dependent on the solution conductivity: a mode in which streamers occurred directly in the liquid (at low conductivities) and a mode in which streamers were generated in a large vapour bubble (at higher conductivities). The thermal effect was suggested by authors as a possible reason for this discharge behaviour due to a higher local heating caused by an increase in Ohmic current with solution conductivity and thus exceeding the threshold energy density required for the formation of a vapour bubble.
Concerning the origin of gas bubbles formed around the electrode tip by the discharge at high pulse repetition rates, they were primarily water vapour bubbles formed by Joule heating of water near the discharge electrode, as was discussed above. In addition, they were also likely the bubbles of molecular hydrogen and oxygen gases produced by plasma chemical processes in water. The chemical analysis of gas content of bubbles in the spheroid was not conducted in this work; however, the formation of H2 and O2 by discharge plasmas in water was determined in many previous studies [5–7]. The bubbles in the spheroid also contained impurities of air dissolved in water. This finding was obtained from experiments with phenol for which nitrated degradation by-products (2- and 4-nitrophenol, 4-nitrocatechol, 4-nitrosophenol) in addition to hydroxylated degradation by-products (catechol, hydroquinone, 1,4-benzoquinone) were detected upon operation of the discharge in bubble mode at high pulse repetition rates, presumably formed by
 and OH· radical attack of phenol ring, respectively (data not shown). Bruggeman et al [26] also observed the presence of nitrogen in bubbles excited by a dc discharge in water. They observed a significant emission from the nitrogen second positive system and the nitrogen ion (first negative system) in the case of the discharge excited in bubble mode, presumably due to the presence of air dissolved in water from the surrounding atmosphere.
and OH· radical attack of phenol ring, respectively (data not shown). Bruggeman et al [26] also observed the presence of nitrogen in bubbles excited by a dc discharge in water. They observed a significant emission from the nitrogen second positive system and the nitrogen ion (first negative system) in the case of the discharge excited in bubble mode, presumably due to the presence of air dissolved in water from the surrounding atmosphere.
Generally, bubbles facilitate breakdown in water since the dielectric strength of gas (∼30 kV cm−1) is much lower than that of water (∼MV cm−1). This fact, however, is in contradiction with the results observed in this work, namely that the bubbles remained in close proximity to the electrode tip and tended to suppress the propagation of the discharge in the form of streamers (even though the initiation of the discharge was slightly faster with higher frequencies; this was probably caused by the presence of bubbles remaining near the electrode tip from the previous discharge periods that facilitated electric breakdown in water from the electrode tip; see figure 2 for comparison of the voltage and current waveforms at pulse repetition rates of 100 and 1000 Hz). This effect became very significant with a pulse repetition rate of approximately 500 Hz, when bubbles near the electrode aggregated together and formed a spheroid composed of a large number of very fine bubbles and surrounded the electrode tip. The process took place very quickly after the application of high-voltage power into the liquid, with all plasmas occurring inside the bubble spheroid. This spheroid (of slightly prolate shape) with a typical radius of about 5 mm was quite stable around the electrode tip, and this was accompanied by random jetting of clusters of small bubbles from the spheroid into the water. Figure 5 shows the images of the bubble spheroid formed in the discharge region and the time evolution of jetting of cluster of small bubbles from the spheroid into the water taken by a high-speed camera during operation of the discharge at a pulse repetition rate of 700 Hz. Figure 5(b) shows that jetting of bubbles from the spheroid was a very violent process accompanied by significant transfer of heat into the surrounding water. This is apparent from figure 5(a), which shows jetting of the bubble cluster using shadowgraph visualization of temperature changes in water around the electrode tip during operation of the discharge (bubble cluster is a round dark mass propagating down from the discharge region).
We assume that the driving force for this process was likely the heat transfer from the discharge into the water. With higher pulse repetition rates more energy from the discharge was converted into heat causing higher and faster increase in vapour pressure inside the bubbles constituting the bubble spheroid around the electrode tip. It is likely that this process eventually led to a violent explosion of the vapour bubble and jetting of cluster of bubbles from the spheroid into the surrounding water. Images taken by the high-speed camera (figure 5(b)) show that the surfaces of bubbles in the clusters were rather rough and irregular indicating rapidly growing bubbles near the limit of superheated liquid. However, direct correlation of the heat transfer with other descriptive parameters for these effects is difficult due to the complex nature of the problem and the different physics contributing to these phenomena whose study was beyond the scope of this work.
Concerning the sustenance of bubble spheroid around the electrode tip during operation of the discharge at high pulse repetition rates we assume that in addition to the thermal effects, surface charging of gas bubbles in the water by a high electric field might contribute to this process. The sustenance of small bubbles in the spheroid (without coalescing them into a single larger bubble) was likely caused by the combined effect of (a) the electrostatic charging of bubbles by an electric field near the electrode tip, which kept these bubbles together, and (b) intense local heating of water near the electrode tip, which prevented small individual bubbles from coalescing into a single larger bubble due to the high non-equilibrium vapour pressure inside these bubbles. An electrical double layer associated with the buildup of surface charge in the bubbles might cause a large change in the electric field distribution in the vicinity of the discharge electrode tip and affect the propagation of streamers in the liquid [27]. With higher pulse repetition rates higher surface charges might be accumulated in the bubbles occurring in the discharge region and the time between the discharge pulses might be too short for relaxation of surface charges accumulated in the bubbles and de-polarization of the electric double layer.
In addition, it is possible that the transition of the discharge characteristics from the streamer mode into the bubble mode was affected by an increase in the solution conductivity of water in the discharge region accompanied by the local heating of water by the discharge. Changes in the temperature and the solution conductivity of water in the discharge region were not directly measured; however, for example, in the case of experiment shown in figure 4, the conductivity of heated KCl solution increased from an initial value of 100 µS cm−1 at 20 °C to above 240 µS cm−1 at 80 °C. Therefore, the solution conductivity might play an important role in the transition process of the discharge into the bubble mode although the changes in temperature and conductivity of water must be local phenomena occurring in the vicinity of the electrode tip since the bulk temperature and conductivity of water in the reactor were maintained constant by the external cooling system (except for experiments shown in figures 4 and 5).
In general, solution conductivity significantly influences the behaviour of a discharge in water [3]. An increase in the solution conductivity is connected with a higher concentration or mobility of ions in the liquid, which strongly alters the propagation of the streamer channel in water by compensating for the space charge electric field on the streamer head. Thus, higher conductivity results, on the one hand, in a larger discharge current due to lower resistivity of liquid media, and, on the other hand, in a shortening of the streamer channel length due to faster compensation of space charge electric field on the streamer head by ions in the liquid. This results in a higher power density in the discharge channel (a larger power dissipated in a smaller volume) resulting in an increase in the plasma density and a higher plasma temperature [2, 3]. It was already mentioned above that solution conductivity influenced the formation of streamer and bubble modes of the discharge in water generated at a pulse frequency of 500 Hz while using low conductive water (1 µS cm−1) or tap water (>100 µS cm−1) [24]. However, in this case the electrical parameters of the discharge were significantly different. The discharge current and load energy consumption of the discharge in low conductive water (1 µS cm−1) were much lower than those in more conductive water (>100 µS cm−1). In this work, no changes in electrical parameters of the discharge in water were observed by varying the pulse repetition rate in the range 50–1000 Hz (i.e. using water with the same initial solution conductivity of 100 µS cm−1). The characteristics of voltage and current waveforms measured for the discharge in the streamer and bubble modes were very similar (see figure 2 for a comparison of the voltage and current waveforms at pulse repetition rates of 100 and 1000 Hz). No significant differences in pulse width, rise time or amplitude were determined and the discharge energy was very similar in both discharge modes (∼250 mJ/pulse). Therefore, a further study of the physics of these phenomena directly in the discharge region is required. The following part of this work presents experimental results on the effects of pulse repetition rate on the chemical and biocidal processes induced by the pulsed discharge in water under different discharge plasma modes.
3.2. Effects of pulse frequency on the chemical activity of the pulsed discharge plasma in water
The effects of pulse repetition rate (i.e. streamer and bubble modes) on the chemical processes induced by the discharge plasma in water were studied through the measurements of H2O2 (hydrogen peroxide) produced by the plasma in water. Hydrogen peroxide is the most abundant chemical species produced by the discharge plasma in water and is mainly formed by the recombination of OH· radicals; it is the sole precursor of OH· radicals formed by the plasma from water primarily by direct electron collisions with water molecules or by several other mechanisms [5, 28]. Although the efficiency of plasma chemical production of H2O2 from water can be affected by many factors [28], hydrogen peroxide is relatively easy to measure and can provide insight into plasma activity. Figure 6 shows the production of hydrogen peroxide generated by the discharge plasma in water in dependence on the pulse repetition rate of the applied voltage. The concentration of H2O2 increased linearly with the number of pulses, indicating a zero-order rate process of H2O2 formation by the plasma at all pulse repetition rates. Formation rates of H2O2 in water
 were higher using lower pulse frequencies (streamer mode) than those obtained using pulse frequencies of 500 Hz and above (bubble mode). The corresponding values of energy efficiency of hydrogen peroxide production
were higher using lower pulse frequencies (streamer mode) than those obtained using pulse frequencies of 500 Hz and above (bubble mode). The corresponding values of energy efficiency of hydrogen peroxide production
 (figure 7) were four times higher in the range 50–100 Hz than for 500–1000 Hz ((8.2–6.9) × 10−9 mol J−1 and (1.9–1.4) × 10−9 mol J−1, respectively). Even in the streamer mode, however, the production of H2O2 significantly varied and continuously decreased with increasing pulse frequency up to 500 Hz. The rates
(figure 7) were four times higher in the range 50–100 Hz than for 500–1000 Hz ((8.2–6.9) × 10−9 mol J−1 and (1.9–1.4) × 10−9 mol J−1, respectively). Even in the streamer mode, however, the production of H2O2 significantly varied and continuously decreased with increasing pulse frequency up to 500 Hz. The rates
 decreased from 2.2 × 10−9 mol/pulse to 0.5 × 10−9 mol/pulse with increasing pulse repetition rate from 50 Hz to 500 Hz, respectively. Using pulse frequencies above 500 Hz the production of H2O2 did not change significantly and similar production rates, (5.0–4.0) × 10−10 mol/pulse, were determined. The decrease in hydrogen peroxide production by the discharge in the streamer mode with increasing pulse frequency was quite surprising. The possible reason for this effect might be the increasing number of bubbles in water, which were produced by the discharge with increasing pulse frequency. These bubbles changed the environment surrounding the electrode tip from the initial pure liquid phase at a low pulse frequency to a gas–liquid phase at a higher pulse frequency. The discharge (although in streamer mode) thus propagated through the increasingly bubbled water in which plasma chemical effects were different from those in the case of the discharge propagated through water without bubbles.
decreased from 2.2 × 10−9 mol/pulse to 0.5 × 10−9 mol/pulse with increasing pulse repetition rate from 50 Hz to 500 Hz, respectively. Using pulse frequencies above 500 Hz the production of H2O2 did not change significantly and similar production rates, (5.0–4.0) × 10−10 mol/pulse, were determined. The decrease in hydrogen peroxide production by the discharge in the streamer mode with increasing pulse frequency was quite surprising. The possible reason for this effect might be the increasing number of bubbles in water, which were produced by the discharge with increasing pulse frequency. These bubbles changed the environment surrounding the electrode tip from the initial pure liquid phase at a low pulse frequency to a gas–liquid phase at a higher pulse frequency. The discharge (although in streamer mode) thus propagated through the increasingly bubbled water in which plasma chemical effects were different from those in the case of the discharge propagated through water without bubbles.
Figure 6. Effect of pulse repetition rate on the formation of hydrogen peroxide by the pulsed discharge in water (20 kV, 100 µS cm−1).
Download figure:
Standard imageFigure 7. Effect of pulse repetition rate on the energy efficiency of hydrogen peroxide production by the pulsed discharge in water (20 kV, 100 µS cm−1).
Download figure:
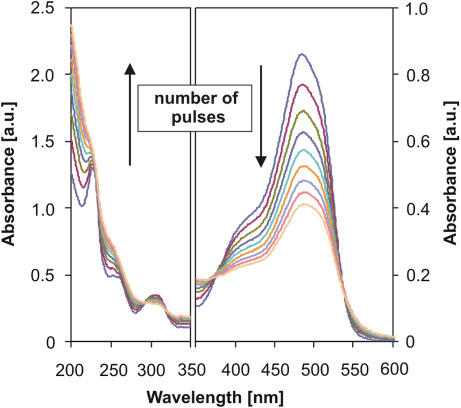
Standard imageThe same effect of streamer and bubble discharge modes was also observed in the degradation of organic dye Acid Orange 7, a model molecule for many azo dyes. The azo group (–N = N–) of this dye is sensitive to oxidation by OH· radicals [6], and AO7 degradation can be easily determined by measuring the fading of the dye colour at the wavelength of maximum absorption (λ = 484 nm). Evaluation of the plasma-induced effects on AO7 was made by measuring the decolourization rate of the dye by the discharge operated with pulse frequencies of 100 and 700 Hz. Figure 8 shows that the decolourization proceeded about 60% faster at the lower frequency, with the rate of 3.2 × 10−6 mg/pulse compared with 1.9 × 10−6 mg/pulse at 700 Hz. Dye degradation obeyed first-order kinetics, which is typical kinetics of electrophilic oxidations. Changes in the absorption spectra of the AO7 molecule (figure 9) indicate that degradation of the dye occurred primarily at the chromophoric azo group and this degradation may be attributed mainly to electrophilic oxidation by OH· radicals.
Figure 8. First-order plot of degradation of organic dye AO7 by the pulsed discharge in water in dependence on the pulse repetition rate of input power (20 kV, 100 µS cm−1).
Download figure:
Standard imageFigure 9. Change of absorption spectra of the dye AO7 with the number of applied discharge pulses (20 kV, 100 Hz, 100 µS cm−1).
Download figure:
Standard imageThese results indicate evidence on the strong dependence of plasma chemical activity (specifically, production of OH· radicals and H2O2) on discharge plasma modes formed in water by varying the pulse frequency of input power (figure 3). It is likely that the reason for this effect might be the different properties of the plasma formed by the discharge in the vapour bubble mode and in the streamer-in-water mode. Previous studies indicate that the optical emission spectra of discharges in bubbles are quite similar to spectra from pulsed streamer discharges in low conductive solutions and consist of OH bands, H, O and atomic lines [7]. However, there are differences in the electron density of the formed plasma. Electron densities reported for discharges excited in vapour bubbles are typically of the order of 1020 m−3 [25], while electron densities up to 1025 m−3 were reported in the case of pulsed streamer discharges in water [3, 29–31]. In addition, as the electric field required for the discharge initiation in vapour bubbles is smaller (∼30 kV cm−1) compared with that for the pulsed streamer discharge plasma in water (∼1 MV cm−1), a lower electron mean energy might be expected in the discharge plasma formed in vapour bubbles. This assumption supports data of Bruggeman et al [26] reporting a smaller electron temperature in the dc-excited discharge plasma formed in the vapour bubble than that in water. The same authors also reported significantly smaller H2O2 production by the discharge formed in the vapour bubble than that in water. Clearly, the magnitude of the temperature of the plasma and the energy distribution of the electrons in the plasma strongly influence the chemical processes induced by the electrical discharge plasma in water [5]. High-energy electrons in the plasma interact with water molecules to produce highly reactive radicals (OH·, H·, O·), which then recombine into more stable molecular species (H2O2, H2, O2). A higher pulse frequency also decreased the contact of discharge plasma with water that also affected the magnitude of chemical and physical processes induced by the plasma in water. These factors might be the reason for the lower degradation of the dye and H2O2 production by the plasma generated in water with higher pulse repetition rates (i.e. the discharge formed in vapour bubbles) compared with that obtained for the plasma generated in water at lower pulse frequencies in streamer discharge mode.
3.3. Effect of pulse frequency on the biocidal activity of the pulsed discharge plasma in water
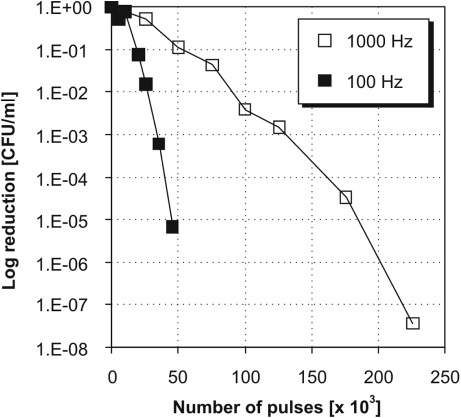
Inactivation of bacteria E. coli by the discharge in water was performed to evaluate the effects of the pulse repetition rate of input power on the biocidal activity of the pulsed discharge in water. High-voltage pulses with pulse repetition rates of 100 and 1000 Hz were selected as experimental conditions for generation of two different modes of discharge plasma in water. Experimental results are shown in figure 10, which shows that the log reduction of E. coli was about four times faster at 100 Hz than at 1000 Hz.
Figure 10. Effect of pulse repetition rate of input power on inactivation of E. coli by the pulsed discharge in water (20 kV, 100 µS cm−1).
Download figure:
Standard imageThese results indicate great variation in the biocidal activity of the discharge plasma in water with pulse repetition rate. Biocidal effects of the plasma in water are largely related to chemical effects induced by the discharge in water. Interaction of chemical species formed in the plasma with bacteria and other cells begins at the surface of the cell, where chemical destruction of the cell membrane and associated components can occur, inducing oxidative stress and eventual death of the cell. In an underwater plasma, physical effects also significantly contribute to the inactivation of bacteria, especially ultraviolet radiation, electric fields or shock waves [32]. It was shown in this paper that the chemical activity of the discharge significantly decreases with a higher pulse repetition rate. There exists an apparent correlation between chemical and biocidal activity of the discharge, which was determined, in both cases, to be about four times lower at high pulse frequencies (<500 Hz) compared with lower pulse frequencies (figures 7 and 10). This indicates that inactivation of E. coli was significantly affected by plasma chemical processes in water also under high frequencies, although the discharge occurred, in this case, largely only in the region of fine bubbles near the electrode tip. Photolysis by UV radiation from the plasma was also likely an important process in E. coli inactivation since the light emission from the discharge plasma was significant even at high pulse frequencies. UV radiation was previously determined to contribute more than 30% to the overall inactivation process induced by pulsed discharge plasmas in water [33]. In addition to the above mentioned processes, we suppose that thermal effects associated with increasing pulse frequency of input power may play an important role in the inactivation of E. coli; however, this effect was not evaluated in this work. We do note that great care was taken to control the temperature of the bacterial suspension during the experiments to minimize heat shock to the bacteria. However, local temperatures in the vicinity of the electrode tip were probably high since large temperature gradients were visually observed in the region close to the electrode tip during operation of the discharge in water with a higher pulse frequency (figure 5(a)). There is also the possibility of synergetic effects of both chemical species and physical processes in the inactivation of bacteria induced by the underwater plasma.
4. Conclusion
Significant effects of the pulse repetition rate of input power on the physical and chemical properties of a pulsed discharge plasma in water were determined. By varying the pulse repetition rate, two distinct modes of discharge plasma were formed in water. The first mode was characterized by the formation of a corona-like discharge propagating through water in the form of streamer channels. The second mode was formed typically above 500 Hz, when the formation of streamer channels in water was suppressed. Here, all plasmas occurred inside a spheroidal aggregate of very fine gas bubbles surrounding the tip of the high-voltage electrode. With higher pulse frequency, thermal processes likely play an important role since input power and contact duration of the plasma with water increased with pulse frequency. The sustenance of bubbles in the spheroid was likely caused by the combined effect of the electrostatic charging of bubbles by an electric field near the electrode tip, which kept these bubbles together, and intense local heating of water near the electrode tip, which prevented small individual bubbles from coalescing into a single larger bubble due to the high non-equilibrium vapour pressure inside these bubbles. With a higher pulse repetition rate more energy from the discharge was converted into heat causing higher and faster increase in vapour pressure inside the bubbles constituting the bubble spheroid around the electrode tip. This process eventually led into the violent explosion of vapour bubble and jetting of cluster of bubbles from the spheroid into the surrounding water. The transition of the discharge characteristics from the streamer mode into the bubble mode was probably also affected by the local increase in the solution conductivity of water in the discharge region, which might alter the propagation of the streamer discharge channel in water by compensating for the space charge electric field on the streamer head. These processes significantly changed the environment surrounding the electrode tip from the initial liquid phase to a gas–liquid phase as well as the chemical and biocidal activity of the discharge in water. The production of hydrogen peroxide, degradation of organic dye Acid Orange 7 and inactivation of bacteria E. coli by the discharge in water decreased significantly using input power with pulse repetition rates above 500 Hz. This decrease was strongly dependent on the discharge plasma modes formed in water by varying the pulse frequency of input power. The reason for this effect was most likely the different properties of the plasma formed directly in water (streamer mode) and in vapour bubble (bubble mode), such as lower electron densities and a lower electron mean energies in plasma discharges excited in vapour bubbles. These parameters strongly influence the formation of chemically reactive species by the plasma through electron collisions with water molecules.
Acknowledgments
This work was supported by the Global COE program on 'Global Initiative Center for Pulsed Power Engineering' of Kumamoto University from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, the Academy of Sciences of the Czech Republic (No M100431203) and the Czech Science Foundation (No 104/09/H080).