Abstract
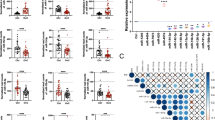
Ovarian cancer as the most fatal gynecological malignancy is often manifested by excessive fluid accumulation known as ascites or effusion. Ascites-derived microRNAs (miRNAs) may be closely associated with ovarian cancer progression. However, our knowledge of their roles, altered expression, and clinical outcomes remained limited. In this study, large-scale expression profiling of 754 human miRNAs was performed using real-time quantitative polymerase chain reaction and 384-well TaqMan array human miRNA A and B cards to identify differentially expressed miRNAs between extracellular fraction of the ascitic fluid associated with high-grade serous ovarian carcinomas and control plasma. Of the 754 miRNAs, 153 were significantly differentially expressed relative to the controls. Expression of 7 individual miRNAs (miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-1290, and miR-30a-5p) was further validated in extended sample sets, including serous, endometrioid, and mucinous subtypes. All miR-200 family members and miR-1290 were conspicuously overexpressed, while miR-30a-5p was only weakly overexpressed. The ability of miRNAs expression to discriminate the pathological samples from the controls was strong. Receiver operating characteristic curve analyses found area under the curve (AUC) values of 1.000 for miR-200a, miR-200c, miR-141, miR-429, and miR-1290 and of AUC 0.996 and 0.885 for miR-200b and miR-30a-5p, respectively. Preliminary survival analyses indicated low expression level of miR-200b as significantly related to longer overall survival (hazard ratio [HR]: 0.25, mean survival 44 months), while high expression level was related to poor overall survival (HR: 4.04, mean survival 24 months). Our findings suggested that ascites-derived miRNAs should be further explored and evaluated as potential diagnostic and prognostic biomarkers for ovarian cancer.
Similar content being viewed by others
References
Matz M, Coleman MP, Carreira H, Salmeron D, Chirlaque MD, Allemani C. Worldwide comparison of ovarian cancer survival: histological group and stage at diagnosis (CONCORD-2). Gynecol Oncol. 2017;144(2):396–404.
Wei W, Li N, Sun Y, Li B, Xu L, Wu L. Clinical outcome and prognostic factors of patients with early stage epithelial ovarian cancer. Oncotarget. 2017;8(14):23862–23870.
Puls LE, Duniho T, Hunter JE, Kryscio R, Blackhurst D, Gallion H. The prognostic implication of ascites in advanced-stage ovarian cancer. Gynecol Oncol. 1996;61(1):109–112.
Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256.
Shender VO, Pavlyukov MS, Ziganshin RH, et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics. 2014;13(12):3558–3571.
van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA. MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol. 2010;42(8):1282–1290.
Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4(3):143–159.
Zuberi M, Khan I, Gandhi G, Ray PC, Saxena A. The conglomeration of diagnostic, prognostic and therapeutic potential of serum miR-199a and its association with clinicopathological features in epithelial ovarian cancer. Tumor Biol. 2016;37(8):11259–11266.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discovery. 2017;16(3):203–221.
Vaksman O, Stavnes HT, Kaern J, Trope CG, Davidson B, Reich R. miRNA profiling along tumour progression in ovarian carcinoma. J Cell Mol Med. 2011;15(7):1593–1602.
Nymoen DA, Slipicevic A, Holth A, et al. MiR-29a is a candidate biomarker of better survival in metastatic high-grade serous carcinoma. Hum Pathol. 2016;54:74–81.
Chung YW, Bae HS, Song JY, et al. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patient. Int J Gynecol Cancer. 2013;23(4):673–679.
Cappellesso R, Tinazzi A, Giurici T, et al. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122(9):685–693.
Vaksman O, Tropę C, Davidson B, Reich R. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis. 2014;35(9):2113–2120.
Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):0034.1.
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Bestkeeper — Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–515.
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2): R19.
Makarova JA, Shkurnikov MU, Wicklein D, et al. Intracellular and extracellular microRNA: an update on localization and biological role. Prog Histochem Cytochem. 2016;51(3–4):33–49.
Weber JA, Baxter DH, Zhang SL, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741.
Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101(10):2087–2092.
Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin Chem. 2015;61(1):56–63.
Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37(11):460–465.
Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360.
Blagden SP. Harnessing pandemonium: the clinical implications of tumor heterogeneity in ovarian cancer. Front Oncol. 2015;5:149.
Nelson BH. New insights into tumor immunity revealed by the unique genetic and genomic aspects of ovarian cancer. Curr Opin Immunol. 2015;33:93–100.
Zavesky L, Jandakova E, Turyna R, et al. New perspectives in diagnosis of gynaecological cancers: emerging role of circulating microRNAs as novel biomarkers. Neoplasma. 2015;62(4):509–520.
Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064.
Jabbari N, Reavis AN, McDonald JF. Sequence variation among members of the miR-200 microRNA family is correlated with variation in the ability to induce hallmarks of mesenchymal—epithelial transition in ovarian cancer cells. J Ovarian Res. 2014;7:12.
Xu S, Xu P, Wu W, et al. The biphasic expression pattern of miR-200a and E-cadherin in epithelial ovarian cancer and its correlation with clinicopathological features. Curr Pharm Des. 2014;20(11):1888–1895.
Choi PW, Ng SW. The functions of microRNA-200 family in ovarian cancer: beyond epithelial—mesenchymal transition. Int J Mol Sci. 2017;18(6):1207.
Kan CWS, Hahn MA, Gard GB, et al. Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer. 2012;12:627.
Gao YC, Wu J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumor Biol. 2015;36(6):4843–4850.
Meng X, Joosse SA, Muller V, et al. Diagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patients. Br J Cancer. 2015;113(9):1358–1366.
Shapira I, Oswald M, Lovecchio J, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110(4):976–983.
Torres A, Torres K, Pesci A, et al. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int J Cancer. 2013;132(7):1633–1645.
Wu J, Ji X, Zhu L, et al. Up-regulation of microRNA-1290 impairs cytokinesis and affects the reprogramming of colon cancer cells. Cancer Lett. 2013;329(2):155–163.
Mao Y, Liu J, Zhang D, Li B. MiR-1290 promotes cancer progression by targeting nuclear factor I/X (NFIX) in esophageal squamous cell carcinoma (ESCC). Biomed Pharmacother. 2015;76:82–93.
Kim G, An HJ, Lee MJ, et al. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer. 2016;91:15–22.
Marchini S, Cavalieri D, Fruscio R, et al. Association between miR-200c and the survival of patients with stage I epithelial ovarian cancer: a retrospective study of two independent tumour tissue collections. Lancet Oncol. 2011;12(3):273–285.
Sestito R, Cianfrocca R, Rosano L, et al. miR-30a inhibits endothelin A receptor and chemoresistance in ovarian carcinoma. Oncotarget. 2016;7(4):4009–4023.
Chen N, Chon HS, Xiong Y, et al. Human cancer cell line microRNAs associated with in vitro sensitivity to paclitaxel. Oncol Rep. 2014;31(1):376–383.
Zhou J, Gong G, Tan H, et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol Rep. 2015;33(6):2915–2923.
Chung YH, Li SC, Kao YH, et al. MiR-30a-5p inhibits epithelial-to-mesenchymal transition and upregulates expression of tight junction protein Claudin-5 in human upper tract urothelial carcinoma cells. Int J Mol Sci. 2017;18(8):1826.
Li Y, Li Y, Chen D, et al. MiR-30a-5p in the tumorigenesis of renal cell carcinoma: a tumor suppressive microRNA. Mol Med Rep. 2016;13(5):4085–4094.
Zhang SL, Liu Q, Zhang Q, Liu L. MicroRNA-30a-5p suppresses proliferation, invasion and tumor growth of hepatocellular cancer cells via targeting FOXA1. Oncol Lett. 2017;14(4):5018–5026.
Zhang BW, Cai HF, Wei XF, et al. MiR-30-5p regulates muscle differentiation and alternative splicing of muscle-related genes by targeting MBNL. Int J Mol Sci. 2016;17(2):E182.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ Note
All patients provided an informed consent. The study was approved by a multicentric ethics committee of the General University Hospital in Prague.
Rights and permissions
About this article
Cite this article
Záveský, L., Jandáková, E., Weinberger, V. et al. Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer. Reprod. Sci. 26, 510–522 (2019). https://doi.org/10.1177/1933719118776808
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118776808