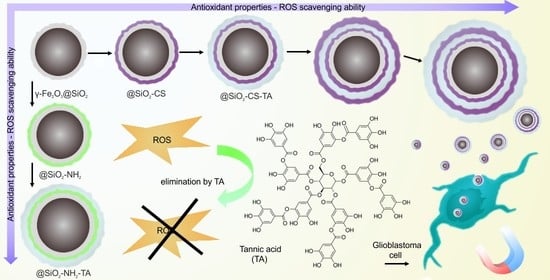
Tannic Acid Coating Augments Glioblastoma Cellular Uptake of Magnetic Nanoparticles with Antioxidant Effects
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of γ-Fe2O3 Nanoparticles and Their Modification with TEOS and APTES
2.3. Modification of γ-Fe2O3@SiO2 Nanoparticles with Chitosan and Tannic Acid
2.4. Physicochemical Characterization of the Particles
2.5. Determination of Cell-Associated Magnetic Nanoparticles (MNPcell)
2.6. Antioxidant Properties of Nanoparticles
2.7. Cellular Toxicity Assay
3. Results and Discussion
3.1. γ-Fe2O3, γ-Fe2O3@SiO2, and γ-Fe2O3@SiO2-NH2 Nanoparticles
3.2. γ-Fe2O3@SiO2-CS-TA Nanoparticles
3.3. γ-Fe2O3@SiO2-NH2-TA Nanoparticles
3.4. Uptake of Differently Coated γ-Fe2O3 Nanoparticles by LN-229 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Sui, X.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug. Deliv. 2020, 27, 1474–1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Sig. Transduct. Target Ther. 2021, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.S.; Packirisamy, M. Cancer-nano-interaction: From cellular uptake to mechanobiological response. Int. J. Mol. Sci. 2021, 22, 9587. [Google Scholar]
- Northcott, J.M.; Dean, I.S.; Mouw, J.K.; Weaver, V.M. Feeling stress: The mechanics of cancer progression and aggression. Front. Cell Dev. Biol. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, M.S.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholts-Sloan, J.S. CBTRUS Statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, 1–100. [Google Scholar] [CrossRef]
- Bae, S.H.; Park, M.J.; Lee, M.M.; Kim, T.M.; Lee, S.H.; Cho, S.Y.; Kim, Y.H.; Kim, Y.J.; Park, C.K.; Kim, C.Y. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J. Korean Med. Sci. 2014, 29, 980–984. [Google Scholar] [CrossRef] [Green Version]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 193–203. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive investigation of the antioxidant and pro-oxidant effects of phenolic compounds: A double-edge sword in the context of oxidative stress? J. Phys. Chem. B. 2018, 122, 6198–6214. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, P.; Liu, C.; Ma, H.; Huang, H.; Lin, Y.; Wang, C.; Yang, Y. pH-Responsive nanodrug encapsulated by tannic acid complex for controlled drug delivery. RSC Adv. 2017, 7, 2829–2835. [Google Scholar] [CrossRef] [Green Version]
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral activity of tannic acid modified silver nanoparticles: Potential to activate immune response in herpes genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(tannic acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Morphological and mechanical properties of tannic acid/PAAm semi-IPN hydrogels for cell adhesion. Polym. Test. 2017, 61, 314–323. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Otrocka-Domagała, I.; Polkowska, I. In vivo studies of novel scaffolds with tannic acid addition. Polym. Degrad. Stab. 2018, 158, 26–30. [Google Scholar] [CrossRef]
- Ding, P.; Wang, Z.; Wu, Z.; Hu, M.; Zhu, W.; Sun, N.; Pei, R. Tannic acid (TA)-functionalized magnetic nanoparticles for EpCAM-independent circulating tumor cell (CTC) isolation from patients with different cancers. ACS Appl. Mater. Interfaces 2021, 13, 3694–3700. [Google Scholar] [CrossRef] [PubMed]
- Atacan, K.; Özacar, M. Characterization and immobilization of trypsin on tannic acid modified Fe3O4 nanoparticles. Colloids Surf. B 2015, 128, 227–236. [Google Scholar] [CrossRef]
- Lu, Y.C.; Luo, P.C.; Huang, C.W.; Leu, Y.L.; Wang, T.H.; Wei, K.C.; Wang, H.E.; Ma, Y.H. Augmented cellular uptake of nanoparticles using tea catechins: Effect of surface modification on nanoparticle–cell interaction. Nanoscale 2014, 6, 10297–10306. [Google Scholar] [CrossRef]
- Cheng, M.C.; Lu, Y.C.; Wu, J.; Ma, Y.H. Gallate-induced nanoparticle uptake by tumor cells: Structure-activity relationships. Colloids Surf. B 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Hafeez, B.B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials 2019, 208, 83–97. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Shahidi, S.B.; Abbasi, M.; Tavakoli, Z.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci. Rep. 2020, 10, 15957. [Google Scholar] [CrossRef]
- Świętek, M.; Lu, Y.C.; Konefał, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.H.; Horák, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1073–1088. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Orainee, B.; Alqudah, D.A.; Daoud, F.; Alshaer, W. Curcumin-tannic acid-poloxamer nanoassemblies enhance curcumin’s uptake and bioactivity against cancer cells in vitro. Int. J. Pharm. 2021, 610, 121255. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Hao, Y.; Xiao, Y.; Qinghai, M. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Kostiv, U.; Janoušková, O.; Šlouf, M.; Kotov, N.; Engstová, H.; Smolková, K.; Ježek, P.; Horák, D. Silica-modified monodisperse hexagonal lanthanide nanocrystals: Synthesis and biological properties. Nanoscale 2015, 7, 18096–18104. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Chang, F.Y.; Tu, S.J.; Chen, J.P.; Ma, Y.H. Cellular uptake of magnetite nanoparticles enhanced by NdFeB magnets in staggered arrangement. J. Magn. Magn. Mater. 2017, 427, 71–80. [Google Scholar] [CrossRef]
- Li, Y.S.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic iron oxide nanoparticles with variable size and an iron oxidation state as prospective imaging agents. Langmuir 2018, 29, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Peternele, W.S.; Fuentes, V.M.; Fascineli, M.L.; Rodrigues da Silva, J.; Silva, R.C.; Lucci, C.M.; Bentes de Azevedo, R. Experimental investigation of the coprecipitation method: An approach to obtain magnetite and maghemite nanoparticles with improved properties. J. Nanomater. 2014, 2014, 682985. [Google Scholar] [CrossRef] [Green Version]
- Świętek, M.; Gunár, K.; Kołodziej, A.; Wesełucha-Birczyńska, A.; Veverka, P.; Šebestová Janoušková, O.; Horák, D. Surface effect of iron oxide nanoparticles on the suppression of oxidative burst in cells. J. Clust. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. App. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Lowe, B.M.; Skylaris, C.K.; Green, N.G. Acid-base dissociation mechanisms and energetics at the silica-water interface: An activationless process. J. Colloid Interface Sci. 2015, 451, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Zasonska, B.A.; Boiko, N.; Klyuchivska, O.; Trchová, M.; Petrovský, E.; Stoika, R.; Horák, D. Silica-coated γ-Fe2O3 nanoparticles: Preparation and engulfment by mammalian macrophages. J. Nanopharm. Drug Deliv. 2013, 1, 182–192. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Shafqat, S.S.; Khan, A.A.; Zafar, M.N.; Alhaji, M.H.; Sanaullah, K.; Shafqat, S.R.; Murtaza, S.; Pang, S.C. Development of amino-functionalized silica nanoparticles for efficient and rapid removal of COD from pre-treated palm oil effluent. J. Mater. Res. Technol. 2019, 8, 385–395. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.J.; Wamer, W.G.; Zeng, M.; Li, Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Baber, O.; Jang, M.; Barber, D.; Powers, K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhal. Toxicol. 2011, 23, 532–543. [Google Scholar] [CrossRef]
- Reczyńska, K.; Marszalek, M.; Zarzycki, A.; Reczyński, W.; Kornaus, K.; Pamuła, E.; Chrzanowski, W. Superparamagnetic iron oxide nanoparticles modified with silica layers as potential agents for lung cancer treatment. Nanomaterials 2020, 10, 1076. [Google Scholar] [CrossRef]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar]
- Shutava, T.G.; Lvov, Y.M. Nano-engineered microcapsules of tannic acid and chitosan for protein encapsulation. J. Nanosci. Nanotechnol. 2006, 6, 1655–1661. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Groberny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Yilmaz, M.D. Layer-by-layer hyaluronic acid/chitosan polyelectrolyte coated mesoporous silica nanoparticles as pH-responsive nanocontainers for optical bleaching of cellulose fabrics. Carbohydr. Polym. 2016, 146, 174–180. [Google Scholar] [CrossRef]
- Ligler, F.S.; Lingerfelt, B.M.; Price, R.P.; Schoen, P.E. Development of uniform chitosan thin-film layers on silicon chips. Langmuir 2001, 17, 5082–5084. [Google Scholar] [CrossRef]
- Valet, S.; Wirth, T.; Höhlinger, M.; Hernándes, Y.T.; Ortiz, J.A.R.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of chitosan/bioactive glass/silica coating on stainless steel and WE43. Surf. Coat. Technol. 2018, 344, 553–563. [Google Scholar]
- An, X.; Kang, Y.; Li, G. The interaction between chitosan and tannic acid calculated based on the density functional theory. Chem. Phys. 2019, 520, 100–107. [Google Scholar] [CrossRef]
- Roy, S.; Zhai, L.; Kim, H.C.; Pham, D.H.; Alrobei, H.; Kim, J. Tannic-acid-cross-linked and TiO2-nanoparticle-reinforced chitosan-based nanocomposite films. Polymers 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Owczarek, A.; Nadolna, K.; Sionkowska, A. The film-forming properties of chitosan with tannic acid addition. Mater. Lett. 2019, 245, 22–24. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, Y.; Wu, Y.; Shi, X.; Du, Y.; Deng, H. Chitosan/tannic acid bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial applications. Inter. J. Biol. Macromol. 2019, 139, 1910198. [Google Scholar] [CrossRef]
- Liao, B.; Xu, C.; Wang, Z.; Li, W.; Liu, X.; Lu, D. Preparation of chitosan-tannic acid coating and its antiosteoclast and antibacterial activities in titanium implant. J. Bone Miner. Metab. Epub 2022, in press. [Google Scholar] [CrossRef]
- Kumorek, M.; Minisy, I.M.; Krunclová, T.; Voršiláková, M.; Venclíková, K.; Mázl Chánová, E.; Janoušková, O.; Kubies, D. pH-responsive and antibacterial properties of self-assembled multilayer films based on chitosan and tannic acid. Mater. Sci. Eng. C 2020, 109, 110493. [Google Scholar] [CrossRef]
- Wahyono, T.; Astuti, D.A.; Wiryawan, K.G.; Sugoro, I.; Jayanegara, A. Fourier transform mid-infrared (FTIR) spectroscopy to identify tannin compounds in the panicle of sorghum mutant lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Liu, J.; Xu, Z. Tannic acid adsorption on amino-functionalized magnetic mesoporous silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, H.; Niu, H.; Ma, X.; Yuan, Y.; Hong, H.; Liu, C. Tannic acid-loaded mesoporous silica for rapid hemostasis and antibacterial activity. Biomater. Sci. 2018, 6, 3318–3331. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhao, W.J.; Yin, H.X.; Lian, H.Z. Facile synthesis of FeIII-tannic acid film-functionalized magnetic silica microspheres for the enrichment of low-abundance peptides and proteins for MALDI-TOF MS analysis. RSC Adv. 2015, 5, 63896. [Google Scholar] [CrossRef]
- Kumar, R.; Mondal, K.; Panda, P.K.; Kaushik, A.; Abolhassani, R.; Ahuja, R.; Rubahn, H.-G.; Mishra, Y.K. Core-shell nanostructures: Perspectives towards drug delivery applications. Mater. Chem. B 2020, 8, 8992–9027. [Google Scholar] [CrossRef] [PubMed]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron oxide mediated photothermal therapy in the second biological window: A comparative study between magnetite/maghemite nanospheres and nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Umh, H.N.; Choi, D.H.; Chao, M.H.; Choi, W.; Kim, S.W.; Kim, Y.; Kim, J.Y. Magnetite- and maghemite-induced different toxicity in murine alveolar macrophage cells. Arch. Toxicol. 2014, 88, 1607–1618. [Google Scholar] [CrossRef]
- Mehrizi, T.Z. Hemocompatibility and hemolytic effects of functionalized nanoparticles on red blood cells: A recent review study. Nano 2021, 16, 213000. [Google Scholar] [CrossRef]





| Particles | Number of Layers | Dh (nm) | PD | ζ-Potential (mV) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| γ-Fe2O3 | 91 | 0.33 | 48 | |||||
| γ-Fe2O3@SiO2 | SiO2 | 228 ± 1 | 0.16 ± 0.004 | −54 ± 0.3 | ||||
| γ-Fe2O3@ SiO2-CS | SiO2 | CS | 226 ± 9 | 0.17 ± 0.02 | 7 ± 0.4 | |||
| γ-Fe2O3@SiO2-CS-TA02 | SiO2 | CS | TA | 212 ± 4 | 0.18 ± 0.01 | −26 ± 1.2 | ||
| γ-Fe2O3@SiO2-CS-TA10 | SiO2 | CS | TA | 218 ± 2 | 0.16 ± 0.01 | −21 ± 1.3 | ||
| γ-Fe2O3@SiO2-CS-TA-CS | SiO2 | CS | TA | CS | 237 ± 12 | 0.19 ± 0.05 | 7 ± 0.2 | |
| γ-Fe2O3@SiO2-CS-TA10/2 | SiO2 | CS | TA | CS | TA | 248 ± 8 | 0.17 ± 0.01 | −20 ± 0.7 |
| γ-Fe2O3@SiO2-NH2 | SiO2 | SiO2-NH2 | 246 ± 2 | 0.16 ± 0.01 | 39 ± 1.2 | |||
| γ-Fe2O3@SiO2-NH2-TA02 | SiO2 | SiO2-NH2 | TA | 248 ± 14 | 0.18 ± 0.05 | −21 ± 1.3 | ||
| γ-Fe2O3@SiO2-NH2-TA10 | SiO2 | SiO2-NH2 | TA | 226 ± 6 | 0.18 ± 0.08 | −25 ± 3.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świętek, M.; Ma, Y.-H.; Wu, N.-P.; Paruzel, A.; Tokarz, W.; Horák, D. Tannic Acid Coating Augments Glioblastoma Cellular Uptake of Magnetic Nanoparticles with Antioxidant Effects. Nanomaterials 2022, 12, 1310. https://doi.org/10.3390/nano12081310
Świętek M, Ma Y-H, Wu N-P, Paruzel A, Tokarz W, Horák D. Tannic Acid Coating Augments Glioblastoma Cellular Uptake of Magnetic Nanoparticles with Antioxidant Effects. Nanomaterials. 2022; 12(8):1310. https://doi.org/10.3390/nano12081310
Chicago/Turabian StyleŚwiętek, Małgorzata, Yunn-Hwa Ma, Nian-Ping Wu, Aleksandra Paruzel, Waldemar Tokarz, and Daniel Horák. 2022. "Tannic Acid Coating Augments Glioblastoma Cellular Uptake of Magnetic Nanoparticles with Antioxidant Effects" Nanomaterials 12, no. 8: 1310. https://doi.org/10.3390/nano12081310