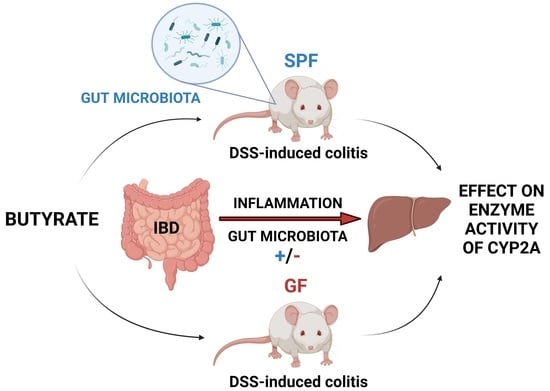
Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Preparation of Microsomal Fraction
4.3. Cytochrome P450 2A Enzyme Activity Assays
4.4. RNA Isolation and Quantitative Real-Time RT-PCR (qRT-PCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Bruneau, A.; Hundertmark, J.; Guillot, A.; Tacke, F. Molecular and Cellular Mediators of the Gut-Liver Axis in the Progression of Liver Diseases. Front. Med. 2021, 8, 725390. [Google Scholar] [CrossRef]
- Iyer, N.; Corr, S.C. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients 2021, 13, 4259. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, V.; Rai, P.; Varshney, S.; Gupta, S.; Khandelwal, N.; Kumar, D.; Nilkanth Gaikwad, A. Sodium butyrate reduces endoplasmic reticulum stress by modulating CHOP and empowers favorable anti-inflammatory adipose tissue immune-metabolism in HFD fed mice model of obesity. Food Chem. 2022, 4, 100079. [Google Scholar] [CrossRef]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2019, 9, 3183. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Antunes, J.C.; Seabra, C.L.; Domingues, J.M.; Teixeira, M.O.; Nunes, C.; Costa-Lima, S.A.; Homem, N.C.; Reis, S.; Amorim, M.T.P.; Felgueiras, H.P. Drug Targeting of Inflammatory Bowel Diseases by Biomolecules. Nanomaterials 2021, 11, 2035. [Google Scholar] [CrossRef]
- Zemanová, N.; Lněničková, K.; Vavrečková, M.; Anzenbacherová, E.; Anzenbacher, P.; Zapletalová, I.; Hermanová, P.; Hudcovic, T.; Kozáková, H.; Jourová, L. Gut microbiome affects the metabolism of metronidazole in mice through regulation of hepatic cytochromes P450 expression. PLoS ONE 2021, 16, e0259643. [Google Scholar] [CrossRef] [PubMed]
- Jourova, L.; Anzenbacher, P.; Anzenbacherova, E. Human gut microbiota plays a role in the metabolism of drugs. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2016, 160, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, T.A.; Baker, D.; Lindon, J.C.; Everett, J.R.; Nicholson, J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 14728–14733. [Google Scholar] [CrossRef] [Green Version]
- Jourová, L.; Vavreckova, M.; Zemanova, N.; Anzenbacher, P.; Langova, K.; Hermanova, P.; Hudcovic, T.; Anzenbacherova, E. Gut Microbiome Alters the Activity of Liver Cytochromes P450 in Mice With Sex-Dependent Differences. Front. Pharmacol. 2020, 11, 01303. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Cui, J.Y.; Klaassen, C.D. RNA-Seq Quantification of Hepatic Drug Processing Genes in Germ-Free Mice. Drug Metab. Dispos. 2015, 43, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Anzenbacher, P.; Anzenbacherová, E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. 2001, 58, 737–747. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Christmas, P. Role of Cytochrome P450s in Inflammation. In Advances in Pharmacology; Hardwick, J.P., Ed.; Academic Press: San Diego, CA, USA, 2015; Volume 74, pp. 163–192. [Google Scholar]
- Pearce, R.E.; Cohen-Wolkowiez, M.; Sampson, M.R.; Kearns, G.L. The role of human cytochrome P450 enzymes in the formation of 2-hydroxymetronidazole: CYP2A6 is the high affinity (low Km) catalyst. Drug Metab. Dispos. 2013, 41, 1686–1694. [Google Scholar] [CrossRef] [Green Version]
- Nitzan, O.; Elias, M.; Peretz, A.; Saliba, W. Role of antibiotics for treatment of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1078–1087. [Google Scholar] [CrossRef]
- Rae, J.M.; Johnson, M.D.; Lippman, M.E.; Flockhart, D.A. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: Studies with cDNA and oligonucleotide expression arrays. J. Pharmacol. Exp. Ther. 2001, 299, 849–857. [Google Scholar]
- Donato, M.T.; Viitala, P.; Rodriguez-Antona, C.; Lindfors, A.; Castell, J.V.; Raunio, H.; Gómez-Lechón, M.J.; Pelkonen, O. CYP2A5/CYP2A6 expression in mouse and human hepatocytes treated with various in vivo inducers. Drug Metab. Dispos. 2000, 28, 1321–1326. [Google Scholar]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Yanagi, K.; Cheng, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Interactions between gut microbiota and non-alcoholic liver disease: The role of microbiota-derived metabolites. Pharmacol. Res. 2019, 141, 521–529. [Google Scholar] [CrossRef]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef]
- Boyapati, R.K.; Dorward, D.A.; Tamborska, A.; Kalla, R.; Ventham, N.T.; Doherty, M.K.; Whitfield, P.D.; Gray, M.; Loane, J.; Rossi, A.G.; et al. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released During Active IBD. Inflamm. Bowel Dis. 2018, 24, 2113–2122. [Google Scholar] [CrossRef]
- Jourova, L.; Satka, S.; Frybortova, V.; Zapletalova, I.; Anzenbacher, P.; Anzenbacherova, E.; Hermanova, P.P.; Drabonova, B.; Srutkova, D.; Kozakova, H.; et al. Butyrate Treatment of DSS-Induced Ulcerative Colitis Affects the Hepatic Drug Metabolism in Mice. Front. Pharmacol. 2022, 13, 936013. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.K. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef]
- Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Regulation of CAR and PXR Expression in Health and Disease. Cells 2020, 9, 2395. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Ikarashi, N.; Hayakawa, Y.; Ishii, M.; Kon, R.; Ochiai, W.; Machida, Y.; Sugiyama, K. Hepatic early inflammation induces downregulation of hepatic cytochrome P450 expression and metabolic activity in the dextran sulfate sodium-induced murine colitis. Eur. J. Pharm. Sci. 2014, 54, 17–27. [Google Scholar] [CrossRef]
- Anzenbacher, P.; Zanger, U.M. Metabolism of Drugs and Other Xenobiotics; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Gerbal-Chaloin, S.; Iankova, I.; Maurel, P.; Daujat-Chavanieu, M. Nuclear receptors in the cross-talk of drug metabolism and inflammation. Drug Metab. Rev. 2013, 45, 122–144. [Google Scholar] [CrossRef]
- Teng, S.; Piquette-Miller, M. The Involvement of the Pregnane X Receptor in Hepatic Gene Regulation during Inflammation in Mice. J. Pharmacol. Exp. Ther. 2005, 312, 841. [Google Scholar] [CrossRef] [Green Version]
- Pascussi, J.M.; Gerbal-Chaloin, S.; Pichard-Garcia, L.; Daujat, M.; Fabre, J.M.; Maurel, P.; Vilarem, M.J. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem. Biophys. Res. Commun. 2000, 274, 707–713. [Google Scholar] [CrossRef]
- Jourova, L.; Anzenbacher, P.; Matuskova, Z.; Vecera, R.; Strojil, J.; Kolar, M.; Nobilis, M.; Hermanova, P.; Hudcovic, T.; Kozakova, H.; et al. Gut microbiota metabolizes nabumetone in vitro: Consequences for its bioavailability in vivo in the rodents with altered gut microbiome. Xenobiotica 2019, 49, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef]
- Jourová, L.; Anzenbacher, P.; Lišková, B.; Matušková, Z.; Hermanová, P.; Hudcovic, T.; Kozáková, H.; Hrnčířová, L.; Anzenbacherová, E. Colonization by non-pathogenic bacteria alters mRNA expression of cytochromes P450 in originally germ-free mice. Folia Microbiol. 2017, 62, 463–469. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Cheng, S.L.; Klaassen, C.D.; Cui, J.Y. Regulation of Hepatic Drug-Metabolizing Enzymes in Germ-Free Mice by Conventionalization and Probiotics. Drug Metab. Dispos. 2016, 44, 262–274. [Google Scholar] [CrossRef]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpiani, D.; Paolo, M.C.D.; Paoluzi, P.; Torsoli, A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M.; et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.-M.; Zhao, H.-L.; Guo, G.-J.; Xu, J.; Zhou, Y.-L.; Huang, H.-L.; Nie, Y.-Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominisand Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Cleophas, M.; Ratter, J.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.; Netea, M.; Joosten, L. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef] [Green Version]
- Jourova, L.; Anzenbacherova, E.; Dostal, Z.; Anzenbacher, P.; Briolotti, P.; Rigal, E.; Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Butyrate, a typical product of gut microbiome, affects function of the AhR gene, being a possible agent of crosstalk between gut microbiome and hepatic drug metabolism. J. Nutr. Biochem. 2022, 107, 109042. [Google Scholar] [CrossRef]
- Little, M.; Dutta, M.; Li, H.; Matson, A.; Shi, X.; Mascarinas, G.; Molla, B.; Weigel, K.; Gu, H.; Mani, S.; et al. Understanding the physiological functions of the host xenobiotic-sensing nuclear receptors PXR and CAR on the gut microbiome using genetically modified mice. Acta Pharm. Sin. B 2022, 12, 801–820. [Google Scholar] [CrossRef]
- Bergan, T.; Bjerke, P.E.M.; Fausa, O. Pharmacokinetics of Metronidazole in Patients with Enteric Disease Compared to Normal Volunteers. Chemotherapy 1981, 27, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Hudcovic, T.; Stĕpánková, R.; Cebra, J.; Tlaskalová-Hogenová, H. The role of microflora in the development of intestinal inflammation: Acute and chronic colitis induced by dextran sulfate in germ-free and conventionally reared immunocompetent and immunodeficient mice. Folia Microbiol. 2001, 46, 565–572. [Google Scholar] [CrossRef]
- Hernández-Chirlaque, C.; Aranda, C.J.; Ocón, B.; Capitán-Cañadas, F.; Ortega-González, M.; Carrero, J.J.; Suárez, M.D.; Zarzuelo, A.; Sánchez de Medina, F.; Martínez-Augustin, O. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J. Crohn’s Colitis 2016, 10, 1324–1335. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.L.M.; Leonel, A.J.; Sad, A.P.; Beltrão, N.R.M.; Costa, T.F.; Ferreira, T.M.R.; Gomes-Santos, A.C.; Faria, A.M.C.; Peluzio, M.C.G.; Cara, D.C.; et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 2012, 23, 430–436. [Google Scholar] [CrossRef]
- Lake, B. Biochemical Toxicology: A Practical Approach; IRL Press: Oxford, UK, 1987; Volume 183. [Google Scholar]
- Schenkman, J.B.; Jansson, I. Spectral Analysis of Cytochromes P450. In Methods Molecular Biology; Phillips, I.R., Shephard, E.A., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 120, pp. 11–18. [Google Scholar]
- Tomankova, V.; Liskova, B.; Skalova, L.; Bartikova, H.; Bousova, I.; Jourova, L.; Anzenbacher, P.; Ulrichova, J.; Anzenbacherova, E. Altered cytochrome P450 activities and expression levels in the liver and intestines of the monosodium glutamate-induced mouse model of human obesity. Life Sci. 2015, 133, 15–20. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satka, S.; Frybortova, V.; Zapletalova, I.; Anzenbacher, P.; Anzenbacherova, E.; Kozakova, H.; Srutkova, D.; Hudcovic, T.; Jourova, L. Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome. Int. J. Mol. Sci. 2022, 23, 11627. https://doi.org/10.3390/ijms231911627
Satka S, Frybortova V, Zapletalova I, Anzenbacher P, Anzenbacherova E, Kozakova H, Srutkova D, Hudcovic T, Jourova L. Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome. International Journal of Molecular Sciences. 2022; 23(19):11627. https://doi.org/10.3390/ijms231911627
Chicago/Turabian StyleSatka, Stefan, Veronika Frybortova, Iveta Zapletalova, Pavel Anzenbacher, Eva Anzenbacherova, Hana Kozakova, Dagmar Srutkova, Tomas Hudcovic, and Lenka Jourova. 2022. "Effect of DSS-Induced Ulcerative Colitis and Butyrate on the Cytochrome P450 2A5: Contribution of the Microbiome" International Journal of Molecular Sciences 23, no. 19: 11627. https://doi.org/10.3390/ijms231911627