Introduction
Adelina spp. (Apicomplexa: Adeleroina: Adeleidae) are parasitic protists of invertebrates, reported to have a worldwide distribution (Berto et al., Reference Berto, Do Bomfim Lopes, Filho, Flausino and Lopes2010). However, knowledge of the diversity of these protists is rather limited, particularly when compared to the diversity of their hosts. In the Canary Islands, an autonomous region of Spain located in the Macaronesian North Atlantic, there are no reports of Adelina spp. On the Iberian Peninsula, insect-related Adeleids have been observed as intra-abdominal oocysts in permanent mounts of sand flies (Morillas-Marquez et al., Reference Morillas-Marquez, Romero-Rodríguez, Ueda-Ontiveros, González-Castro and Guevara-Benítez1983; Martinez-Ortega and Conesa-Gallego, Reference Martinez-Ortega and Conesa-Gallego1987). These have only been identified to genus level which is understandable considering the large overlap in morphological parameters which exists between most of the described species (Purrini, Reference Purrini1984; Berto et al., Reference Berto, Do Bomfim Lopes, Filho, Flausino and Lopes2010).
The pathogenicity of these protozoa has not been studied extensively in natural invertebrate communities, however, their capacity to contribute to species competition, behavioural and colour changes, paralysis, darkening of internal organs and ultimately as a cause of death, have been demonstrated (Table 1). Thus, in addition to their likely natural role in population regulation, there may be a role for Adelina spp. as a means of biological pest control in farming (Yarwood, Reference Yarwood1937; Park and Frank, Reference Park and Frank1950; Weisner, Reference Weisner1964; Purrini, Reference Purrini1984; El-Sufty and Boraei, Reference El-Sufty and Boraei1989).
Table 1. Recorded pathological effects of Adelina spp. on arthropod species around the world under laboratory or natural (Lab/Nat) conditions

Adelina spp. are currently divided into two lineages; one group is found in the body cavity, while the second includes gut parasites. Classically, the genus Adelina (body cavity parasites) was erected from Adelea spp. (intestinal parasites), with differentiation of the two genera based on morphology of the sporocysts, which are spherical and discoidal, respectively (Yarwood, Reference Yarwood1937). Based on these morphological features, several species from Adelea and Klossia were reclassified within the genus Adelina. However, with the exception of Adelina dimidiata and A. schellacki, which infect myriapods, all Adelina spp. are body cavity parasites (Purrini, Reference Purrini1984). Few molecular genetic studies have been undertaken in this genus, however comparing available sequences from NCBI (accession numbers in brackets), the difference of 4.3% between A. dimidiata (DQ096835.1) and Adelina grylli (body cavity) (DQ096836.2) is greater than other apicomplexans such as Cystoisospora canis (KT184368.1) compared with Toxoplasma gondii (2.2%, V03070.1;KX008033.1), Neospora caninum (1.9%, L24380.1) or Besnoitia spp. (B. darlingi (1.8%) MF872603.1; B. besnoiti (1.5%) XR_003828658.1). Further research is clearly needed to refine the current taxonomical status of these species and thus the intestinal infecting Adelina species are not considered further in this review.
The life cycle of Adelina spp. occurs inside the arthropod body cavity, with sporozoites piercing the gut to access the coelom (Merritt et al., Reference Merritt, Thomas and Christensen1975). Asexual division takes place, forming two generations of merogonies (as described for A. cryptocerci) followed, after release of the merozoites into fatty tissue, by sexual reproduction of gametoblasts (Yarwood, Reference Yarwood1937). These macro and microgametoblasts fuse and develop into a zygote, which finally forms a sporont (Yarwood, Reference Yarwood1937; Park and Frank, Reference Park and Frank1950; Ghosh et al., Reference Ghosh, Choudhury and Misra2000). Sporulation generally occurs within the fat bodies. As the infection spreads, the body tries to encapsulate the oocysts within tissue, to isolate them, and these appear as dark aggregates (Park and Frank, Reference Park and Frank1950; El-Sufty and Boraei, Reference El-Sufty and Boraei1989). Finally, the adeleids begin to occupy the majority of the coelom and the rest of organs including muscles, resulting in death of the insect (Bhatia, Reference Bhatia1937; Park and Frank, Reference Park and Frank1950; El-Sufty and Boraei, Reference El-Sufty and Boraei1989). Other authors report secondary infections with gut bacteria as a cause of death in invertebrates, after penetration through the gut wall by the coccidia (Merritt et al., Reference Merritt, Thomas and Christensen1975).
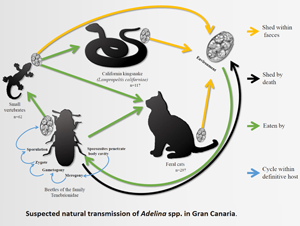
To infect other hosts, the oocysts must be released to the environment and then be ingested by other invertebrates. This can happen by cannibalism or through a ‘dispersion host’ (Sautet, Reference Sautet1930; Butaeva, Reference Butaeva1996; De Quadros et al., Reference De Quadros, De Moura, Rodrigues, Antonelli and Veronezi2017). A dispersion host is typically a vertebrate predator which ingests an invertebrate whose tissues contain Adelina oocysts, and which are then released into its digestive tract and excreted. This phenomenon has been observed in several vertebrate species (reptiles, amphibians, birds and mammals), in which the parasite-infected invertebrates form part of their diet (Barnard et al., Reference Barnard, Ernst and Dixon1974; Berto et al., Reference Berto, Lopes, Flausino, Teixeira Filho and Lopes2008; Lopes et al., Reference Lopes, Spitz dos Santos, Ribeiro Luz, Pereira Berto and Gomes Lopes2013; De Quadros et al., Reference De Quadros, De Moura, Rodrigues, Antonelli and Veronezi2017).
The Canary Islands are an archipelago composed by eight islands and five islets in Macaronesia. Despite their small size (7447 km2), the Canaries are home to one of the largest number of endemic species in the temperate regions globally (Machado, Reference Machado1998). Among the varied landscapes of the islands, which are considered ‘hot-spots’ of biodiversity, the laurel forests are particularly unique, found only in Macaronesia (Machado, Reference Machado1998). Even considering their small size, there are between 2 and 5 isoclimatic zones, depending on the island, with four in the case of Gran Canaria: dry desert, dry steppe, temperate mild and temperate cold (Rodríguez-Ponce et al., Reference Rodríguez-Ponce, Molina and Hernández1995).
On Gran Canaria, 5872 species of flora and fauna have been recorded to date, of which 22.7% are considered endemic. Arthropods comprise the largest and most diverse group with 3190 species recorded to date, of which 32.1% are endemic to the island (Arechavaleta et al., Reference Arechavaleta, Rodríguez, Zurita and García2010). Although arthropods constitute more than half the total species described on the island, there is a total dearth of knowledge of their coccidian parasites or their potential role in the regulation of arthropod populations within the islands. Moreover, considering the introduction of foreign parasitic species into the islands by exotic arthropods [612 introduced species and 66 invasive species. (Arechavaleta et al., Reference Arechavaleta, Rodríguez, Zurita and García2010)], an evaluation of current invertebrate parasites present on the island is much needed.
This study aims to contribute to baseline data for studies on invertebrate parasites in Macaronesia, their dissemination hosts as well as documenting the oocysts found.
Materials and methods
Between 2016 and 2019, faecal samples from various vertebrate animal species from Gran Canaria were analysed at the Laboratory of Parasitology, Faculty of Veterinary Sciences of the University of Las Palmas de Gran Canaria.
Faecal samples from cats were obtained from live animals during a larger study of feral cat colonies from across the island and donated from neutering release campaigns. For the remaining animals, the faeces were collected during post-mortem examination of fresh or frozen carcasses. The animals were obtained from the Tafira Wildlife Recovery Centre (naturally dead hedgehogs and birds) or Gestion y Planeamiento Territorial y Medioambiental (GesPlan) who conduct the eradication programme of invasive California kingsnakes (Lampropeltis californiae) in Gran Canaria. The samples from dogs were obtained during post-mortem examination of animals from the local animal shelter (Albergue insular de animales, Arucas) during practical classes in the Veterinary Faculty.
For species others than dogs and cats, all the collected faeces were used for concentration methods. For small amounts of sample, a minimum quantity of 0.5 mL of faeces were placed in each of three microcentrifuge tubes for processing. Samples with less than 0.5m L were discarded. For cats and dogs an average of 1.5 g of faeces were used for each concentration test. All faecal samples were tested for parasites using flotation in saturated sodium chloride solution (density 1.2 g mL−1), zinc sulphate centrifugal flotation (density 1.18 g mL−1) and formol-ether concentration method (7 parts of 10% formalin, 3 parts of pure diethyl-ether) (Willis, Reference Willis1921; Faust et al., Reference Faust, D'Antoni, Odom, Miller, Peres, Sawitz, Thomen, Tobie and Walker1938; Zajac and Conboy, Reference Zajac, Conboy, Zajac and Conboy2012). Proper parasites and pseudoparasites were recorded.
The identification was carried by using the available references for pseudoparasitic elements in vertebrate faeces (Parker and Duszynski, Reference Parker and Duszynski1986; Berto et al., Reference Berto, Lopes, Flausino, Teixeira Filho and Lopes2008; Lopes et al., Reference Lopes, Spitz dos Santos, Ribeiro Luz, Pereira Berto and Gomes Lopes2013; De Quadros et al., Reference De Quadros, De Moura, Rodrigues, Antonelli and Veronezi2017).
From each positive sample, oocysts were measured using a calibrated microscope (Leitz Laborlux S).
Results
In all, 476 faecal samples from 298 feral cats, 117 California kingsnakes, 10 Algerian hedgehogs (Atelerix algirus caniculus), 15 feral dogs and 36 birds from seven species were examined. Of these birds, many were species endemic to Macaronesia (M) or subspecies endemic to the Canary Islands (C) and included 10 Turdus merula, 9 Falco tinnunculus canariensis (C), 8 Asio otus canariensis (C), 3 Passer hispaniolensis, 3 Serinus canaria (M), 2 Apus unicolor (M) and 1 Gallinula chloropus.
Of the 476 samples, just four contained round to slightly ellipsoidal oocysts containing more than 4 (6–16) round sporocysts, consistent with the definition of the genus Adelina. These positive samples were from one cat, from the municipality of La Aldea de San Nicolás, in the west of the island; and three snakes from the municipality of Telde in the east giving a total Adelina spp. oocyst prevalence of 0.8% (4/476) across all samples, and 0.3% (1/298) and 2.6% (3/117) of feral cat and snake samples respectively. Measurements of oocysts and sporocysts in from each species are presented in Table 2 and compared with the other Adelina species described in the literature (Purrini, Reference Purrini1984).
Table 2. Measurements of the stages of the parasite are given [meront (M), macrogametocyte (Ma), microgametocyte (Mi), and oocyst (O)], to summarize and facilitate the identification of future Adelina spp. in histological sections, fresh invertebrate tissues or as pseudoparasites in faeces

Adelina spp. described, but thus far un-named, have not been considered. All the measurements are in micrometres. S, sporocyst; NS, number of sporocysts. In the author column the first one is the original description, authors in brackets are the source of the description represented in this table. If only an author in brackets is cited, represent also the original description.
Based on the size of the oocysts and sporocysts, the coccidia in the cat faeces resembled Adelina picei (two oocysts) (Fig. 1A), but the number of sporocysts found in these specimens was 6–8, while that described for A. picei is 8–18.

Fig. 1. Photomicrographs of sporulated Adelina spp. oocysts. (A) A. picei from a feral cat. (B) A. tribolii from snake 1. (C) A. tribolii from snake 2. (D) A. tribolii from snake 3. Scale bars = 20 μm.
The coccidia from snake no. 1 (three oocysts) (Fig. 1B), were considered to be Adelina tribolii-like species, as the measurements and morphology (41 × 28–29 μm oocysts, slightly ellipsoidal 11 × 10–11 μm sporocysts, 8–9 sporocysts per oocyst) fell within the ranges of A. tribolii [26–50 × 22–36 μm oocysts, round sporocysts 10.4 μm and 2–24 sporocysts per oocyst (Purrini, Reference Purrini1984)]. In the faeces from snake no. 2 (two oocysts) (Fig. 1C), the coccidia most closely resembled A. tribolii based on the size of the oocysts and the number of sporocysts. Finally, the coccidia found in the faeces of snake no. 3 (two oocysts) (Fig. 1D) are possibly the same species as in snake no. 1 i.e. A. tribolii-like oocysts, but with slightly bigger sporocysts.
Discussion
In a diagnostic laboratory, pseudoparasitic elements, as well as pollen grains, fungal spores and yeasts, dust mite eggs and even fly larvae are usually present in faecal samples at the time of analysis. With experience, the technician can distinguish what is and what is not a parasitic element. However, in the case of carnivorous animals these pseudoparasitic elements could be parasites of their prey species. Frequently these prey parasites are disrupted and may appear ‘dead’, but in the case of Adelina the eggs survive inside the bowel of the predator (dispersion host) and are disseminated to the environment with the faeces, in the same way ingested plant seeds would also be dispersed.
The results of this study indicate the presence of at least two species of Adelina resembling A. tribolii and A. picei on the island of Gran Canaria. However, morphological measures of the oocysts are close to several reported species, but with potentially important differences in sporocyst numbers (Table 2). This fact may be important from the perspective of the identification of very similar species by molecular methods, considering the huge variation in A. tribolii sporocysts (from 2 to 24). This variation could be also explained by the process of sporulation, with two sporocysts being erroneously reported as mature oocysts, instead of 24, or the presence of several cryptic species. In addition, the lack of further ecological, morphological and molecular data from the actual definitive host, leave the speciation just presumptive at this stage.
California kingsnakes, unlike cats, are not known to eat invertebrates and thus the presence of adeleids in the faeces of a non-insectivorous snake could be explained through their regular prey on Gran Canaria: the Gran Canaria giant lizard (Gallotia stehlini), geckos (Tarentola boettgeri), skinks (Chalcides sexlineatus) and rodents (Monzón-Argüello et al., Reference Monzón-Argüello, Patiño-Martínez, Christiansen, Gallo-Barneto, Cabrera-Pérez, Peña-Estévez, López-Jurado and Lee2015). These prey species usually consume arthropods and thus the oocysts may have originated from invertebrates within their gastrointestinal tract. In support of this theory is the finding, in the snake faeces, of other parasites from these prey reptile species such as eggshells of Pharyngodonidae oxiurids.
Despite all species in this study having a diet which includes insects, neither species of Adelina spp. was found. A possible explanation, given the low prevalence obtained from snakes and cats, could be the sample size of each species, as well as the scarcity of faeces in small animals. Furthermore, the accurate diet composition of the other species of the study could also influence the species of Adelina to be found e.g. swifts (Apus spp.) prey on tiny flying insects caught on the wing which may not contain Adelina spp.. Previous studies on wild invertebrates demonstrate a prevalence of Adelina spp. between 3 and 27% (Merritt et al., Reference Merritt, Thomas and Christensen1975; El-Sufty and Boraei, Reference El-Sufty and Boraei1986, Reference El-Sufty and Boraei1989). What is not clear is if the low prevalence studies can be explained by selection failure of the sampled arthropods, due to death of infected immature stages. Considering the wide prevalence variation reported in other studies, it is not clear if the low figure of 0.8% in this study, is truly representative of the overall prevalence of Adelina in Gran Canaria. These two vertebrate species (cats and snakes) could amplify the number of oocysts in faeces by consuming more prey such as geckoes, serving as sentinel species for Adelina spp. surveys. Further studies are required to more accurately determine the prevalence of Adelina within definitive and other dispersion hosts.
Although data are scarce, Adeleid coccidia could be considered important ecosystem ‘regulators’, causing death of various arthropod species (Table 1). Under laboratory conditions, 20% fewer larval stages are reported vs non-infected insects, demonstrating how insect populations, can be influenced by these parasites (Park and Frank, Reference Park and Frank1950). Insects which are resistant to Adelina spp. have a significant selective advantage over those which are non-resistant (Park and Frank, Reference Park and Frank1950; Lange and Lord, Reference Lange, Lord, Vega and Kaya2012). Without the selective pressure of the parasite, the non-resistant insects dominate over the resistant ones.
The presence of Adelina spp. in stool samples from vertebrates is important from an ecological point of view, as digestion by vertebrates is required to release the oocysts from the invertebrate tissues, and disseminate within their faeces (Parker and Duszynski, Reference Parker and Duszynski1986; De Quadros et al., Reference De Quadros, De Moura, Rodrigues, Antonelli and Veronezi2017). This has been widely studied in other parts of the world with Adeleorid coccidia demonstrated in vertebrate faeces as pseudoparasites (Parker and Duszynski, Reference Parker and Duszynski1986; Berto et al., Reference Berto, Lopes, Flausino, Teixeira Filho and Lopes2008; Lopes et al., Reference Lopes, Spitz dos Santos, Ribeiro Luz, Pereira Berto and Gomes Lopes2013; De Quadros et al., Reference De Quadros, De Moura, Rodrigues, Antonelli and Veronezi2017). Indeed, a genus of coccidia (Pythonella spp.) was erroneously described as a reptile parasite when it is actually a pseudoparasite (Kawazoe and Gouvêa, Reference Kawazoe and Gouvêa1999; Ghimire, Reference Ghimire2010).
Dispersion hosts, on occasion, travel long distances or even, in the case of migratory birds, may move from one country or region to another, disseminating their parasites to their new habitat. This phenomenon has been widely demonstrated in ticks, with tick-borne diseases being carried from one country to another (Hasle, Reference Hasle2013). Furthermore, novel parasites introduced by these dispersion hosts or by exotic/invasive invertebrates may cause more significant disease in naïve invertebrate hosts than the natural infected host populations (Kelehear and Jones, Reference Kelehear and Jones2010; Bacela-Spychalska et al., Reference Bacela-Spychalska, Wattier, Genton and Rigaud2012; Martín-Torrijos et al., Reference Martín-Torrijos, Campos Llach, Pou-Rovira and Diéguez-Uribeondo2017). However, host specificity and thus the real impact of Adelina spp. in natural invertebrate populations, compared with laboratory populations, is not currently understood. Neither co-invasion nor host switch in natural insect populations infected with Adelina spp. has been reported in the literature, thus, further research is needed. Indeed, Gran Canaria, with its huge invertebrate diversity could be considered an ideal model island system to study this and other invertebrate parasites, starting with morphological and molecular surveys, and promotion of conservation programmes.
In general terms, coccidian parasites, including Adelina spp., are very host specific, affecting mostly animals from the same genus. Adelina tribolii has been described in three species of flour beetles (Tribolium spp.) (Table 1) (Park and Frank, Reference Park and Frank1950), a genus of beetle from the family Tenebrionidae. Based on this, A. tribolii-like records from Gran Canaria are most-likely parasites of a Tribolium sp., possibly the invasive species red flour beetle (T. castaneum) or confused flour beetle (T. confusum) which are the only known species recorded on the island. The other putative species recorded in this study, Adelina picei has been reported parasitizing Alphitobius sp., another tenebrionid beetle. Considering host specificity related to the genus of the host, for Adelina picei another two beetle species could be suitable hosts in Gran Canaria: the introduced lesser mealworm (A. diaperinus) and the black fungus beetle (A. laevigatus).
The definitive host species of the Adelina pseudoparasites remains unknown, however cats are known to consume Tenebrionid beetles often in feral life, unlike L. californiae (Medina and Nogales, Reference Medina and Nogales2009; Monzón-Argüello et al., Reference Monzón-Argüello, Patiño-Martínez, Christiansen, Gallo-Barneto, Cabrera-Pérez, Peña-Estévez, López-Jurado and Lee2015; Gallo-Barneto et al., Reference Gallo-Barneto, Cabrera-Pérez, Peña-Estevez, Patiño-Martinez and Monzón-Argüello2016). Based on this data, Adelina could be present in Tenebrionids, of which several species are endemic and endangered (Arechavaleta et al., Reference Arechavaleta, Rodríguez, Zurita and García2010). Further sampling would be needed, in conjunction with molecular work, to address the accurate epidemiology of this parasite in Gran Canaria and other parts of the world.
Conclusions
Despite a low prevalence, these findings constitute the first baseline data for invertebrate pathology studies in the Canary Islands. Further epidemiological research on invertebrate parasites in these islands would be necessary to determine the invertebrate hosts, native or exotic, and the real epidemiological importance of insectivorous animals in the life cycle of Adelina spp. The further understanding of the role of this protozoan in invertebrate population dynamics is particularly important in an island setting where the vast majority of fauna is native/endemic and/or endangered. The Canaries, and other similar islands, could be utilized as model systems for arthropod parasites. Using morphological measures, the oocysts described here are close to several reported species, but with potentially important differences in sporocyst numbers. Further material should be studied to determine its accurate taxonomical status, considering the morphological variability of A. tribolii. With the appropriate molecular sampling of Adeleids within invertebrates, the vertebrate species studied here could be useful as sentinels for further research on Adelina spp. in the Canary Islands and further afield.
Acknowledgements
The authors would like to thank the collaboration of Ramón Gallo Barneto, Head of Gestión y planeamiento territorial y ambiental (GesPlan S.A.) as well as Miguel Ángel Cabrera Pérez, from Servicio de Biodiversidad, Dirección general de protección de la naturaleza, Gobierno de Canarias and Pascual Calabuig for the donation of the specimens, to the personnel of GesPlan, who collected snakes in the field and finally, Mr. de Blas for his help with photography and graphic content.
Author contribution
Kevin M. Santana-Hernández and Eligia Rodríguez-Ponce conceived and designed the study. Kevin M. Santana-Hernández and Eligia Rodríguez-Ponce conducted data gathering. Kevin M. Santana-Hernández, Simon L. Priestnall, David Modrý and Eligia Rodríguez-Ponce wrote the article.
Financial support
This study was supported by the project ‘POSTLIFE + Lampropeltis para el control de la culebra real de California en Gran Canaria (LIFE10/NAT/ES/656)’ financed by the Government of Canary Islands and Cabildo of Gran Canaria.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.