Abstract
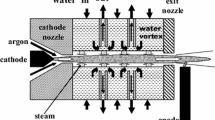
Reactions of methane with water and CO2 in thermal plasma generated in a special plasma torch with a water-stabilized arc were investigated. Steam plasma with very high enthalpy and low mass flow rate was produced in a dc arc discharge which was in direct contact with water vortex surrounding the arc column. Composition of produced gas, energy balance of the process and its efficiency were determined from measured data. The output H2/CO ratio could be adjusted by a choice of feed rates of input reactants in the range 1.1–3.4. Depending on experimental conditions the conversion of methane was up to 99.5%, the selectivity of H2 was up to 99.9%, and minimum energy needed for production of 1 mol of hydrogen was 158 kJ/mol. Effect of conditions on process characteristics was studied. Comparison of measured data with results of theoretical computations confirmed that the reforming process produces gas with composition which is close to the one obtained from the thermodynamic equilibrium calculations. Relations between process enthalpy, composition of produced syngas and process characteristics were determined both theoretically and experimentally.
















Similar content being viewed by others
References
Holladay JD, Hu J, King DL, Wang Y (2009) An overview of hydrogen production technologies. Catal Today 139:244–260
Lubitz W, Tumas B (2007) Hydrogen: an overview. Chem Rev 107:3900–3903
Oyama ST, Hacarlioglu P, Gu Y, Lee D (2012) Dry reforming of methane has no future for hydrogen production: comparison with steam reforming at high pressure in standard and membrane reactors. Int J Hydrogen Energy 37:10444–10450
Ryi S, Park J, Kim D, Kim T, Kim S (2009) Methane steam reforming with a novel catalytic nickel membrane for effective hydrogen production. J Membr Sci 339:189–194
Wilhelm DJ, Simbeck DR, Karp AD, Dickenson RL (2001) Syngas production for gas-to-liquids applications: technologies, issues and outlook. Fuel Process Technol 71:139–148
Petitpas G, Rolliera JD, Darmonb A, Gonzalez-Aguilara J, Metkemeijera R, Fulcheri L (2007) A comparative study of non-thermal plasma assisted reforming technologies. Int J Hydrogen Energy 32:2848–2867
Zou JJ, Zhang YP, Liu CJ, Li Y, Eliasson B (2003) Starch-enhanced synthesis of oxygenates from methane and carbon dioxide using dielectric-barrier discharge. Plasma Chem Plasma Process 23:69–82
Song HK, Lee H, Choi JW, Na BK (2004) Effect of electrical pulse forms on the CO2 reforming of methane using atmospheric dielectric barrier discharge. Plasma Chem Plasma Process 24:57–72
Zhang YP, Li Y, Wang Y, Liu CJ, Eliasson B (2003) Plasma methane conversion in the presence of carbon dioxide using dielectric-barrier discharges. Fuel Process Technol 83:101–109
Christophe DB, Tom M, Jan VD, Sabine P, Bert V, Steven C, Annemie B (2011) Dielectric barrier discharges used for the conversion of greenhouse gases: modeling the plasma chemistry by fluid simulations. Plasma Sources Sci Technol 20:1–11
Dai B, Zhang XL, Gong WM, He R (2000) Study on the methane coupling under pulse corona plasma by using CO2 as oxidant. Plasma Sci Technol 2:577–580
Li MW, Xu GH, Tian YL, Chen L, Fu HF (2004) Carbon dioxide reforming of methane using DC corona discharge plasma reaction. J Phys Chem A 108:1687–1693
Chen Q, Dai W, Tao XM et al (2006) CO2 reforming of CH4 in abnormal glow plasma under atmospheric pressure. Plasma Sci Technol 8:5–7
Kalra CS, Gutsol AF, Fridman AA (2005) Gliding arc discharge as a source of intermediate plasma for methane partial oxidation. IEEE Trans Plasma Sci 33:32–34
Tu X, Whitehead JC (2014) Plasma dry reforming of methane in an atmospheric pressure AC gliding arc discharge: co-generation of syngas and carbon nanomaterials. Int J Hydrogen Energy 39:9658–9669
Xu GF, Ding XW (2012) Optimization geometries of a vortex gliding-arc reactor for partial oxidation of methane. Energy 47:337–339
Morgan NN, ElSabbagh M (2017) Hydrogen production from methane through pulsed DC plasma. Plasma Chem Plasma Process 37:1375–1392
Wang YF, Tsai CH, Chang WY, Kuo YM (2010) Methane steam reforming for producing hydrogen in an atmospheric pressure microwave plasma reactor. Int J Hydrogen Energy 35:135–140
Jasinski M, Dors M, Nowakowska H, Nichipor GV, Mizeraczyk J (2011) Production of hydrogen via conversion of hydrocarbons using a microwave plasma. J Phys D Appl Phys 44(194002):7
Choi Dae Hyun, Chun Se Min, Maa Suk Hwal, Hong Yong Cheol (2016) Production of hydrogen-rich syngas from methane reforming by steam microwave plasma. J Ind Eng Chem 34:286–291
Sekiguchi H, Mori Y (2003) Steam plasma reforming using microwave discharge. Thin Solid Films 435:44–48
Tianyang Li T, Rehmet Ch, Cheng Y, Jin Y, Cheng Y (2017) Experimental comparison of methane pyrolysis in thermal plasma. Plasma Chem Plasma Process 37:1033–1049
Rutberg PG, Kuznetsov VA, Popov VE, Popov SD, Surov AV, Subbotin DI, Bratsev AN (2015) Conversion of methane by CO2 + H2O + CH4 plasma. Appl Energy 148:159–168
Ni G, Lan Y, Cheng C, Meng Y, Wang X (2011) Reforming of methane and carbon dioxide by DC water plasma at atmospheric pressure. Int J Hydrogen Energy 36:12869–12876
Tao X, Bai M, Wu Q, Huang Z, Yin Y, Dai X (2009) CO2 reforming of CH4 by bianode thermal plasma. Int J Hydrogen Energy 34:9373–9378
Tao X, Qi F, Yin Y, Dai X (2008) CO2 reforming of CH4 by combination of thermal plasma and catalyst. Int J Hydrogen Energy 33:1262–1265
Chase MW Jr (ed) (1998) NIST-JANAF thermochemical tables, 4th edn. American Chemical Society and American Institute of Physics, New York
Hrabovsky M, Hlina M, Konrad M, Kopecky V, Chumak O, Kavka T, Maslani A (2009) Thermal plasma gasification of biomass for fuel gas production. High Temp Mater Process 13:299–313
Hrabovsky M, Hlina M, Kopecky V, Maslani A, Zivny O, Krenek P, Serov A, Hurba O (2017) Steam plasma treatment of organic substances for hydrogen and syngas production. Plasma Chem Plasma Process 37:739–762
Agon N, Hrabovsky M, Chumak O, Hlina M, Kopecky V, Maslani A, Bosmans A, Helsen L, Skoblja S, Van Oost G, Vierendeels J (2016) Plasma gasification of refuse derived fuel in a single-stage system using different gasifying agents. Waste Manag 47:246–255
Hrabovsky M, Kopecky V, Sember V, Kavka T, Chumak O, Konrad M (2006) Properties of hybrid water/gas DC arc plasma torch. IEEE Trans Plasma Sci 34:1566–1575
Hrabovsky M (2002) Generation of thermal plasmas in liquid and hybrid DC arc torches. Pure Appl Chem 74:429–433
U.S. Department of Energy (DOE) https://www.hydrogen.energy.gov/index.html
Acknowledgements
The authors gratefully acknowledge the financial support of the Grant Agency of the Czech Republic under the project GA15-19444S and 17-10246J.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrabovsky, M., Hlina, M., Kopecky, V. et al. Steam Plasma Methane Reforming for Hydrogen Production. Plasma Chem Plasma Process 38, 743–758 (2018). https://doi.org/10.1007/s11090-018-9891-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-018-9891-5